Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex
- PMID: 15728193
- PMCID: PMC2171818
- DOI: 10.1083/jcb.200408157
Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex
Abstract
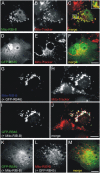
The ribbon complex of retinal photoreceptor synapses represents a specialization of the cytomatrix at the active zone (CAZ) present at conventional synapses. In mice deficient for the CAZ protein Bassoon, ribbons are not anchored to the presynaptic membrane but float freely in the cytoplasm. Exploiting this phenotype, we dissected the molecular structure of the photoreceptor ribbon complex. Identifiable CAZ proteins segregate into two compartments at the ribbon: a ribbon-associated compartment including Piccolo, RIBEYE, CtBP1/BARS, RIM1, and the motor protein KIF3A, and an active zone compartment including RIM2, Munc13-1, a Ca2+ channel alpha1 subunit, and ERC2/CAST1. A direct interaction between the ribbon-specific protein RIBEYE and Bassoon seems to link the two compartments and is responsible for the physical integrity of the photoreceptor ribbon complex. Finally, we found the RIBEYE homologue CtBP1 at ribbon and conventional synapses, suggesting a novel role for the CtBP/BARS family in the molecular assembly and function of central nervous system synapses.
Figures






References
-
- Altrock, W.D., S. tom Dieck, M. Sokolov, A.C. Meyer, A. Sigler, C. Brakebusch, R. Fässler, K. Richter, T.M. Boeckers, H. Potschka, et al. 2003. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein Bassoon. Neuron. 37:787–800. - PubMed
-
- Brandstätter, J.H., E.L. Fletcher, C.C. Garner, E.D. Gundelfinger, and H. Wässle. 1999. Differential expression of the presynaptic cytomatrix protein Bassoon among ribbon synapses in the mammalian retina. Eur. J. Neurosci. 11:3683–3693. - PubMed
-
- Brose, N., K. Hofmann, Y. Hata, and T.C. Südhof. 1995. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C-domain protein. J. Biol. Chem. 270:25273–25280. - PubMed
-
- Calakos, N., S. Schoch, T.C. Südhof, and R.C. Malenka. 2004. Multiple roles for the active zone protein RIM1α in late stages of neurotransmitter release. Neuron. 42:889–896. - PubMed
-
- Cases-Langhoff, C., B. Voss, A.M. Garner, U. Appeltauer, K. Takei, S. Kindler, R.W. Veh, P. De Camilli, E.D. Gundelfinger, and C.C. Garner. 1996. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. Eur. J. Cell Biol. 69:214–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

