Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy
- PMID: 15728237
- PMCID: PMC2213068
- DOI: 10.1084/jem.20041367
Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy
Abstract
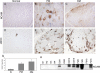
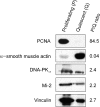
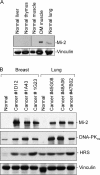
Unique autoantibody specificities are strongly associated with distinct clinical phenotypes, making autoantibodies useful for diagnosis and prognosis. To investigate the mechanisms underlying this striking association, we examined autoantigen expression in normal muscle and in muscle from patients with autoimmune myositis. Although myositis autoantigens are expressed at very low levels in control muscle, they are found at high levels in myositis muscle. Furthermore, increased autoantigen expression correlates with differentiation state, such that myositis autoantigen expression is increased in cells that have features of regenerating muscle cells. Consistent with this, we found that cultured myoblasts express high levels of autoantigens, which are strikingly down-regulated as cells differentiate into myotubes in vitro. These data strongly implicate regenerating muscle cells rather than mature myotubes as the source of ongoing antigen supply in autoimmune myositis. Myositis autoantigen expression is also markedly increased in several cancers known to be associated with autoimmune myositis, but not in their related normal tissues, demonstrating that tumor cells and undifferentiated myoblasts are antigenically similar. We propose that in cancer-associated myositis, an autoimmune response directed against cancer cross-reacts with regenerating muscle cells, enabling a feed-forward loop of tissue damage and antigen selection. Regulating pathways of antigen expression may provide unrecognized therapeutic opportunities in autoimmune diseases.
Figures




References
-
- von Muhlen, C.A., and E.M. Tan. 1995. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin. Arthitis Rheum. 24:323–358. - PubMed
-
- Plotz, P.H. 2003. The autoantibody repertoire: searching for order. Nat. Rev. Immunol. 3:73–78. - PubMed
-
- Targoff, I.N., and M. Reichlin. 1985. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 28:796–803. - PubMed
-
- Love, L.A., R.L. Leff, D.D. Fraser, I.N. Targoff, M. Dalakas, P.H. Plotz, and F.W. Miller. 1991. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 70:360–374. - PubMed
-
- Arnett, F.C., T.J. Hirsch, W.B. Bias, M. Nishikai, and M. Reichlin. 1981. The Jo-1 antibody system in myositis: relationships to clinical features and HLA. J. Rheumatol. 8:925–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

