Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2
- PMID: 15728241
- PMCID: PMC2213053
- DOI: 10.1084/jem.20041611
Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2
Abstract
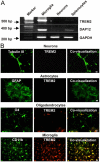
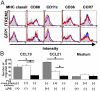
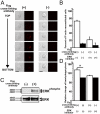
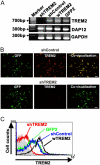
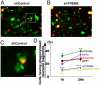
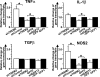
Elimination of apoptotic neurons without inflammation is crucial for brain tissue homeostasis, but the molecular mechanism has not been firmly established. Triggering receptor expressed on myeloid cells-2 (TREM2) is a recently identified innate immune receptor. Here, we show expression of TREM2 in microglia. TREM2 stimulation induced DAP12 phosphorylation, extracellular signal-regulated kinase phosphorylation, and cytoskeleton reorganization and increased phagocytosis. Knockdown of TREM2 in microglia inhibited phagocytosis of apoptotic neurons and increased gene transcription of tumor necrosis factor alpha and nitric oxide synthase-2, whereas overexpression of TREM2 increased phagocytosis and decreased microglial proinflammatory responses. Thus, TREM2 deficiency results in impaired clearance of apoptotic neurons and inflammation that might be responsible for the brain degeneration observed in patients with polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy/Nasu-Hakola disease.
Figures



References
-
- Savill, J., I. Dransfield, C. Gregory, and C. Haslett. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2:965–975. - PubMed
-
- Lauber, K., S.G. Blumenthal, M. Waibel, and S. Wesselborg. 2004. Clearance of apoptotic cells: getting rid of the corpses. Mol. Cell. 14:277–287. - PubMed
-
- Voll, R.E., M. Herrmann, E.A. Roth, C. Stach, J.R. Kalden, and I. Girkontaite. 1997. Immunosuppressive effects of apoptotic cells. Nature. 390:350–351. - PubMed
-
- Fadok, V.A., D.L. Bratton, A. Konowal, P.W. Freed, J.Y. Westcott, and P.M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101:890–898. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

