CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells
- PMID: 15728465
- PMCID: PMC1403291
- DOI: 10.4049/jimmunol.174.5.2591
CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells
Abstract
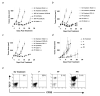
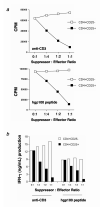
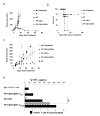
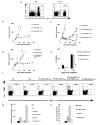
CD4(+) T cells control the effector function, memory, and maintenance of CD8(+) T cells. Paradoxically, we found that absence of CD4(+) T cells enhanced adoptive immunotherapy of cancer when using CD8(+) T cells directed against a persisting tumor/self-Ag. However, adoptive transfer of CD4(+)CD25(-) Th cells (Th cells) with tumor/self-reactive CD8(+) T cells and vaccination into CD4(+) T cell-deficient hosts induced autoimmunity and regression of established melanoma. Transfer of CD4(+) T cells that contained a mixture of Th and CD4(+)CD25(+) T regulatory cells (T(reg) cells) or T(reg) cells alone prevented effective adoptive immunotherapy. Maintenance of CD8(+) T cell numbers and function was dependent on Th cells that were capable of IL-2 production because therapy failed when Th cells were derived from IL-2(-/-) mice. These findings reveal that Th cells can help break tolerance to a persisting self-Ag and treat established tumors through an IL-2-dependent mechanism, but requires simultaneous absence of naturally occurring T(reg) cells to be effective.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

