Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development
- PMID: 15728671
- PMCID: PMC2835538
- DOI: 10.1242/dev.01719
Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development
Abstract
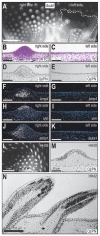
The avian feather complex represents a vivid example of how a developmental module composed of highly integrated molecular and histogenic programs can become rapidly elaborated during the course of evolution. Mechanisms that facilitate this evolutionary diversification may involve the maintenance of plasticity in developmental processes that underlie feather morphogenesis. Feathers arise as discrete buds of mesenchyme and epithelium, which are two embryonic tissues that respectively form dermis and epidermis of the integument. Epithelial-mesenchymal signaling interactions generate feather buds that are neatly arrayed in space and time. The dermis provides spatiotemporal patterning information to the epidermis but precise cellular and molecular mechanisms for generating species-specific differences in feather pattern remain obscure. In the present study, we exploit the quail-duck chimeric system to test the extent to which the dermis regulates the expression of genes required for feather development. Quail and duck have distinct feather patterns and divergent growth rates, and we exchange pre-migratory neural crest cells destined to form the craniofacial dermis between them. We find that donor dermis induces host epidermis to form feather buds according to the spatial pattern and timetable of the donor species by altering the expression of members and targets of the Bone Morphogenetic Protein, Sonic Hedgehog and Delta/Notch pathways. Overall, we demonstrate that there is a great deal of spatiotemporal plasticity inherent in the molecular and histogenic programs of feather development, a property that may have played a generative and regulatory role throughout the evolution of birds.
Figures



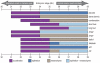
References
-
- Alberch P. Problems with the interpretation of developmental sequences. System. Zool. 1985;34:46–58.
-
- Albrecht UEG, Helms JA, Lin H. Visualization of gene expression patterns by in situ hybridization. In: Daston GP, editor. Molecular and Cellular Methods in Developmental Toxicology. CRC Press; Boca Raton, FL: 1997. pp. 23–48.
-
- Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int. J. Dev. Biol. 2002;46:243–253. - PubMed
-
- Atit R, Conlon RA, Niswander L. EGF signaling patterns the feather array by promoting the interbud fate. Dev. Cell. 2003;4:231–240. - PubMed
-
- Bee J, Thorogood P. The role of tissue interactions in the skeletogenic differentiation of avian neural crest cells. Dev. Biol. 1980;78:47–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

