Identification and characterization of a novel alpha-kinase with a von Willebrand factor A-like motif localized to the contractile vacuole and Golgi complex in Dictyostelium discoideum
- PMID: 15728726
- PMCID: PMC1087232
- DOI: 10.1091/mbc.e04-07-0639
Identification and characterization of a novel alpha-kinase with a von Willebrand factor A-like motif localized to the contractile vacuole and Golgi complex in Dictyostelium discoideum
Abstract
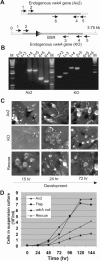
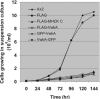
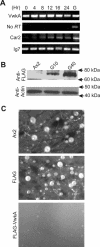
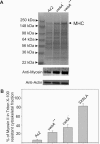
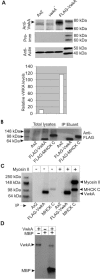
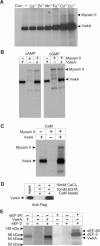
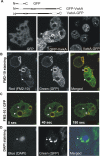
We have identified a new protein kinase in Dictyostelium discoideum that carries the same conserved class of "alpha-kinase" catalytic domain as reported previously in myosin heavy chain kinases (MHCKs) in this amoeba but that has a completely novel domain organization. The protein contains an N-terminal von Willebrand factor A (vWFA)-like motif and is therefore named VwkA. Manipulation of VwkA expression level via high copy number plasmids (VwkA++ cells) or gene disruption (vwkA null cells) results in an array of cellular defects, including impaired growth and multinucleation in suspension culture, impaired development, and alterations in myosin II abundance and assembly. Despite sequence similarity to MHCKs, the purified protein failed to phosphorylate myosin II in vitro. Autophosphorylation activity, however, was enhanced by calcium/calmodulin, and the enzyme can be precipitated from cellular lysates with calmodulin-agarose, suggesting that VwkA may directly bind calmodulin. VwkA is cytosolic in distribution but enriched on the membranes of the contractile vacuole and Golgi-like structures in the cell. We propose that VwkA likely acts indirectly to influence myosin II abundance and assembly behavior and possibly has broader roles than previously characterized alpha kinases in this organism, which all seem to be MHCKs.
Figures



References
-
- Aarts, M., Iihara, K., Wei, W. L., Xiong, Z. G., Arundine, M., Cerwinski, W., MacDonald, J. F., and Tymianski, M. (2003). A key role for TRPM7 channels in anoxic neuronal death. Cell 115, 863–877. - PubMed
-
- Adachi, H., Hasebe, T., Yoshinaga, K., Ohta, T., and Sutoh, K. (1994). Isolation of Dictyostelium discoideum cytokinesis mutants by restriction enzyme-mediated integration of the blasticidin S resistance marker. Biochem. Biophys. Res. Commun. 205, 1808–1814. - PubMed
-
- Betapudi, V., Shoebotham, K., and Egelhoff, T. T. (2004). Generation of double gene disruptions in Dictyostelium discoideum using a single antibiotic marker selection. Biotechniques 36, 106–112. - PubMed
-
- Buxton, D. B., Golomb, E., and Adelstein, R. S. (2003). Induction of nonmuscle myosin heavy chain II-C by butyrate in RAW 264.7 mouse macrophages. J. Biol. Chem. 278, 15449–15455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

