Mechanism of action of T-705 against influenza virus
- PMID: 15728892
- PMCID: PMC549233
- DOI: 10.1128/AAC.49.3.981-986.2005
Mechanism of action of T-705 against influenza virus
Abstract
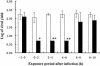
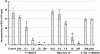
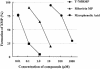
T-705, a substituted pyrazine compound, has been found to exhibit potent anti-influenza virus activity in vitro and in vivo. In a time-of-addition study, it was indicated that T-705 targeted an early to middle stage of the viral replication cycle but had no effect on the adsorption or release stage. The anti-influenza virus activity of T-705 was attenuated by addition of purines and purine nucleosides, including adenosine, guanosine, inosine, and hypoxanthine, whereas pyrimidines did not affect its activity. T-705-4-ribofuranosyl-5'-triphosphate (T-705RTP) and T-705-4-ribofuranosyl-5'-monophosphate (T-705RMP) were detected in MDCK cells treated with T-705. T-705RTP inhibited influenza virus RNA polymerase activity in a dose-dependent and a GTP-competitive manner. Unlike ribavirin, T-705 did not have an influence on cellular DNA or RNA synthesis. Inhibition of cellular IMP dehydrogenase by T-705RMP was about 150-fold weaker than that by ribavirin monophosphate, indicating the specificity of the anti-influenza virus activity and lower level of cytotoxicity of T-705. These results suggest that T-705RTP, which is generated in infected cells, may function as a specific inhibitor of influenza virus RNA polymerase and contributes to the selective anti-influenza virus activity of T-705.
Figures


References
-
- Birch, G. M. 1995. The intracellular formation of a mononucleotide of the anti-influenza agent 1,3,4-thiadiazol-2-ylcyanamide (LY217896). Antivir. Chem. Chemother. 6:127-137.
-
- Cheer, S. M., and A. J. Wagstaff. 2002. Spotlight on zanamivir in influenza. Am. J. Respir. Med. 1:147-152. - PubMed
-
- Crotty, S., D. Maag, J. J. Arnold. W. Zhong, J. Y. N. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. - PubMed
-
- Fernandez, H. 1986. Ribavirin: a clinical overview. Eur. J. Epidemiol. 2:1-14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

