In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication
- PMID: 15728900
- PMCID: PMC549243
- DOI: 10.1128/AAC.49.3.1039-1045.2005
In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication
Abstract
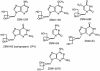
We have reported previously that methylenecyclopropane analogs of nucleosides have excellent activity against certain members of the herpesvirus family. A second generation, the 2,2-bis-hydroxymethyl derivatives, were synthesized, and 18 compounds were tested for activity in vitro against herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), human and murine cytomegalovirus (HCMV and MCMV), varicella-zoster virus (VZV), and Epstein-Barr virus (EBV). Selected analogs were also evaluated against human herpesvirus type 6 (HHV-6) and HHV-8. None of the 18 compounds had activity against HSV-1 or HSV-2, but four were active against VZV by plaque reduction (PR) assay at 50% effective concentration (EC(50)) levels of < or =50 microM. Six of the 18 compounds were active against HCMV by cytopathic effect or PR assays with EC(50)s of 0.5 to 44 microM, and all were active against MCMV by PR (0.3 to 54 microM). Four of the compounds were active against EBV by enzyme-linked immunosorbent assay (<0.3 to 4.4 microM). Four compounds with CMV activity were also active against HHV-6A and HHV-6B (0.7 to 28 microM), and three compounds were active against HHV-8 (5.5 to 16 microM). One of these, ZSM-I-62, had particularly good activity against CMV, HHV-6, and HHV-8, with EC(50)s of 0.7 to 8 microM. Toxicity was evaluated in adherent and nonadherent cells, and minimal cytotoxicity was observed. Mechanism of action studies with HCMV suggested that these compounds are phosphorylated by the ppUL97 phosphotransferase and are potent inhibitors of viral DNA synthesis. These results indicate that at least one of these compounds may have potential for use in treating CMV and other herpesvirus infections in humans.
Figures

Similar articles
-
Efficacy of methylenecyclopropane analogs of nucleosides against herpesvirus replication in vitro.Nucleosides Nucleotides Nucleic Acids. 2003 Dec;22(12):2105-19. doi: 10.1081/ncn-120026633. Nucleosides Nucleotides Nucleic Acids. 2003. PMID: 14714760
-
Synthesis and antiviral activity of (Z)- and (E)-2,2-[bis(hydroxymethyl)cyclopropylidene]methylpurines and -pyrimidines: second-generation methylenecyclopropane analogues of nucleosides.J Med Chem. 2004 Jan 29;47(3):566-75. doi: 10.1021/jm030316s. J Med Chem. 2004. PMID: 14736238
-
Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro.Antimicrob Agents Chemother. 2005 Sep;49(9):3724-33. doi: 10.1128/AAC.49.9.3724-3733.2005. Antimicrob Agents Chemother. 2005. PMID: 16127046 Free PMC article.
-
The UL97 protein kinase of human cytomegalovirus and homologues in other herpesviruses: impact on virus and host.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):169-80. doi: 10.1016/j.bbapap.2003.11.022. Biochim Biophys Acta. 2004. PMID: 15023359 Review.
-
Acyclic/carbocyclic guanosine analogues as anti-herpesvirus agents.Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):271-85. doi: 10.1081/NCN-100002298. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11563039 Review.
Cited by
-
Cytomegalovirus infection lengthens the cell cycle of granule cell precursors during postnatal cerebellar development.JCI Insight. 2024 Jun 10;9(11):e175525. doi: 10.1172/jci.insight.175525. JCI Insight. 2024. PMID: 38855871 Free PMC article.
-
Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro.Antimicrob Agents Chemother. 2006 Apr;50(4):1564-6. doi: 10.1128/AAC.50.4.1564-1566.2006. Antimicrob Agents Chemother. 2006. PMID: 16569886 Free PMC article.
-
Cyclopropavir susceptibility of cytomegalovirus DNA polymerase mutants selected after antiviral drug exposure.Antimicrob Agents Chemother. 2012 Jan;56(1):197-201. doi: 10.1128/AAC.05559-11. Epub 2011 Oct 3. Antimicrob Agents Chemother. 2012. PMID: 21968367 Free PMC article.
-
TAOK3 phosphorylates the methylenecyclopropane nucleoside MBX 2168 to its monophosphate.Antiviral Res. 2015 Jul;119:23-7. doi: 10.1016/j.antiviral.2015.04.001. Epub 2015 Apr 7. Antiviral Res. 2015. PMID: 25857706 Free PMC article.
-
Highlights in antiviral drug research: antivirals at the horizon.Med Res Rev. 2013 Nov;33(6):1215-48. doi: 10.1002/med.21256. Epub 2012 May 2. Med Res Rev. 2013. PMID: 22553111 Free PMC article. Review.
References
-
- Baldanti, F., A. Sarasini, J. C. Drach, J. Zemlicka, and G. Gerna. 2002. Z-isomers of 2-hydroxymethylcyclopropylidenemethyl adenine (synadenol) and guanine (synguanol) are active against ganciclovir- and foscarnet-resistant human cytomegalovirus UL97 mutants. Antiviral Res. 56:273-278. - PubMed
-
- Bidanset, D. J., C. B. Hartline, D. J. Collins, D. C. Quenelle, Y. Chen, J. Zemlicka, and E. R. Kern. 2002. In vitro and in vivo activity of the methylenecyclopropane analogs Qyl-1064, against murine and human cytomegalovirus infection. Antiviral Res. 53:A61.
-
- Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. - PMC - PubMed
-
- Boivin, G., C. Gilbert, A. Gaudreau, I. Greenfield, R. Sudlow, and N. A. Roberts. 2001. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J. Infect. Dis. 184:1598-1602. - PubMed
-
- Britt, W. A., and C. A. Alford. 2001. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, P. M. Howley, and D. E. Griffin (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

