Synthesis and antileishmanial activities of novel 3-substituted quinolines
- PMID: 15728905
- PMCID: PMC549264
- DOI: 10.1128/AAC.49.3.1076-1080.2005
Synthesis and antileishmanial activities of novel 3-substituted quinolines
Abstract
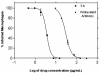
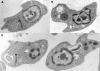
The antileishmanial efficacy of four novel quinoline derivatives was determined in vitro against Leishmania chagasi, using extracellular and intracellular parasite models. When tested against L. chagasi-infected macrophages, compound 3b demonstrated 8.3-fold greater activity than did the standard pentavalent antimony. No significant activity was found for compounds 3a, 4a, and 4b. The antilesihmanial effect of compound 3b was independent of host cell activation, as demonstrated by nitric oxide production. Ultrastructural studies of promastigotes treated with compound 3b showed mainly enlarged mitochondria, with matrix swelling and reduction in the number of cristae. Synthetic analogues based on the quinoline ring structure, already an established template for antiparasitic drugs, could provide further useful compounds.
Figures

References
-
- Amougay, A., O. Letsch, and J. P. Pete. 1996. Photorearrangement of N- alkanoyl β-enaminones. Application to the synthesis of α-amino-β,γ-unsaturated acid derivatives. Tetrahedron 52:2405-2420.
-
- Antonioletti, R., F. Bonadies, L. R. Orelli, and A. Scettri. 1992. Selective C- alkylation of 1,3-dicarbonyl compounds. Gazz. Chim. Ital. 122:237-238.
-
- Balaña-Fouce, R., R. M. Reguera, C. Cubria, and D. Ordóñez. 1998. The pharmacology of leishmaniasis. Gen. Pharmacol. 30:435-443. - PubMed
-
- Balaraman, S., P. Tewary, V. K. Singh, and R. Madhubala. 2004. Leishmania donovani induces interferon regulatory factor in murine macrophages: a host defense response. Biochem. Biophys. Res. Commun. 30:639-647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

