Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus
- PMID: 15728913
- PMCID: PMC549257
- DOI: 10.1128/AAC.49.3.1127-1134.2005
Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus
Abstract
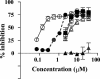
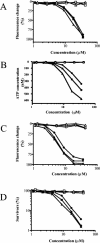
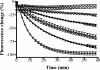
The emergence and spread of multidrug-resistant gram-positive bacteria represent a serious clinical problem. Telavancin is a novel lipoglycopeptide antibiotic that possesses rapid in vitro bactericidal activity against a broad spectrum of clinically relevant gram-positive pathogens. Here we demonstrate that telavancin's antibacterial activity derives from at least two mechanisms. As observed with vancomycin, telavancin inhibited late-stage peptidoglycan biosynthesis in a substrate-dependent fashion and bound the cell wall, as it did the lipid II surrogate tripeptide N,N'-diacetyl-L-lysinyl-D-alanyl-D-alanine, with high affinity. Telavancin also perturbed bacterial cell membrane potential and permeability. In methicillin-resistant Staphylococcus aureus, telavancin caused rapid, concentration-dependent depolarization of the plasma membrane, increases in permeability, and leakage of cellular ATP and K(+). The timing of these changes correlated with rapid , concentration-dependent loss of bacterial viability, suggesting that the early bactericidal activity of telavancin results from dissipation of cell membrane potential and an increase in membrane permeability. Binding and cell fractionation studies provided direct evidence for an interaction of telavancin with the bacterial cell membrane; stronger binding interactions were observed with the bacterial cell wall and cell membrane relative to vancomycin. We suggest that this multifunctional mechanism of action confers advantageous antibacterial properties.
Figures

References
-
- Allen, N. E., D. L. LeTourneau, and J. N. Hobbs, Jr. 1997. The role of hydrophobic side chains as determinants of antibacterial activity of semisynthetic glycopeptide antibiotics. J. Antibiot. (Tokyo) 50:677-684. - PubMed
-
- Allen, N. E., and T. I. Nicas. 2003. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol. Rev. 26:511-532. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

