Ex vivo induction of viral antigen-specific CD8 T cell responses using mRNA-electroporated CD40-activated B cells
- PMID: 15730391
- PMCID: PMC1809302
- DOI: 10.1111/j.1365-2249.2005.02733.x
Ex vivo induction of viral antigen-specific CD8 T cell responses using mRNA-electroporated CD40-activated B cells
Abstract
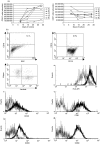
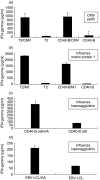
Cell-based immunotherapy, in which antigen-loaded antigen-presenting cells (APC) are used to elicit T cell responses, has become part of the search for alternative cancer and infectious disease treatments. Here, we report on the feasibility of using mRNA-electroporated CD40-activated B cells (CD40-B cells) as alternative APC for the ex vivo induction of antigen-specific CD8(+) T cell responses. The potential of CD40-B cells as APC is reflected in their phenotypic analysis, showing a polyclonal, strongly activated B cell population with high expression of MHC and co-stimulatory molecules. Flow cytometric analysis of EGFP expression 24 h after EGFP mRNA-electroporation showed that CD40-B cells can be RNA transfected with high gene transfer efficiency. No difference in transfection efficiency or postelectroporation viability was observed between CD40-B cells and monocyte-derived dendritic cells (DC). Our first series of experiments show clearly that peptide-pulsed CD40-B cells are able to (re)activate both CD8+ and CD4(+) T cells against influenza and cytomegalovirus (CMV) antigens. To demonstrate the ability of viral antigen mRNA-electroporated CD40-B cells to induce virus-specific CD8+ T cell responses, these antigen-loaded cells were co-cultured in vitro with autologous peripheral blood mononuclear cells (PBMC) for 7 days followed by analysis of T cell antigen-specificity. These experiments show that CD40-B cells electroporated with influenza M1 mRNA or with CMV pp65 mRNA are able to activate antigen-specific interferon (IFN)-gamma-producing CD8(+) T cells. These findings demonstrate that mRNA-electroporated CD40-B cells can be used as alternative APC for the induction of antigen-specific (memory) CD8(+) T cell responses, which might overcome some of the drawbacks inherent to DC immunotherapy protocols.
Figures


Similar articles
-
Simultaneous activation of viral antigen-specific memory CD4+ and CD8+ T-cells using mRNA-electroporated CD40-activated autologous B-cells.J Immunother. 2006 Sep-Oct;29(5):512-23. doi: 10.1097/01.cji.0000210385.48327.1e. J Immunother. 2006. PMID: 16971807
-
RNA-electroporated CD40-activated B cells induce functional T-cell responses against HepG2 cells.Eur J Cancer Care (Engl). 2008 Jul;17(4):404-11. doi: 10.1111/j.1365-2354.2007.00841.x. Eur J Cancer Care (Engl). 2008. PMID: 18485013
-
Autogeneic rna-electroporated CD40-ligand activated b-cells from hepatocellular carcinoma patients induce CD8+ T-cell responses ex vivo.Exp Oncol. 2007 Jun;29(2):137-43. Exp Oncol. 2007. PMID: 17704747
-
CD40-activated B cells as antigen-presenting cells: the final sprint toward clinical application.Expert Rev Vaccines. 2013 Jun;12(6):631-7. doi: 10.1586/erv.13.39. Expert Rev Vaccines. 2013. PMID: 23750793 Review.
-
Cancer immunotherapy using RNA-loaded dendritic cells.Clin Exp Immunol. 2003 Dec;134(3):378-84. doi: 10.1046/j.1365-2249.2003.02286.x. Clin Exp Immunol. 2003. PMID: 14632740 Free PMC article. Review.
Cited by
-
Langerhans-type and monocyte-derived human dendritic cells have different susceptibilities to mRNA electroporation with distinct effects on maturation and activation: implications for immunogenicity in dendritic cell-based immunotherapy.J Transl Med. 2013 Jul 9;11:166. doi: 10.1186/1479-5876-11-166. J Transl Med. 2013. PMID: 23837662 Free PMC article.
-
Optimizing the process of nucleofection for professional antigen presenting cells.BMC Res Notes. 2015 Sep 24;8:472. doi: 10.1186/s13104-015-1446-8. BMC Res Notes. 2015. PMID: 26404473 Free PMC article.
-
B cell-regulated immune responses in tumor models and cancer patients.Oncoimmunology. 2013 Jul 1;2(7):e25443. doi: 10.4161/onci.25443. Oncoimmunology. 2013. PMID: 24073382 Free PMC article. Review.
-
Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression.Clin Cancer Res. 2011 Aug 1;17(15):4987-95. doi: 10.1158/1078-0432.CCR-11-0207. Epub 2011 Jun 20. Clin Cancer Res. 2011. PMID: 21690573 Free PMC article.
-
B lymphocytes as effector cells in the immunotherapy of cancer.J Surg Oncol. 2012 Mar 15;105(4):431-5. doi: 10.1002/jso.22093. Epub 2011 Sep 6. J Surg Oncol. 2012. PMID: 21898417 Free PMC article. Review.
References
-
- Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–9. - PubMed
-
- Zhou Y, Bosch ML, Salgaller ML. Current methods for loading dendritic cells with tumor antigen for induction of antitumor immunity. J Immunother. 2002;25:289–303. - PubMed
-
- Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. - PubMed
-
- Rini BI. Technology evaluation. APC-8015, Dendreon. Curr Opin Mol Ther. 2002;4:76–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

