Cytokine signals through STAT3 promote expression of granulocyte secondary granule proteins in 32D cells
- PMID: 15730854
- PMCID: PMC2388245
- DOI: 10.1016/j.exphem.2004.11.014
Cytokine signals through STAT3 promote expression of granulocyte secondary granule proteins in 32D cells
Abstract
Objective: In a previous study, we showed that activation of a transfected human erythropoietin receptor (EPOR) in the murine myeloid cell line 32D resulted in the development of morphologic features of granulocytic differentiation and expression of the neutrophil primary granule protein myeloperoxidase. We now studied if EPOR signaling could also mediate secondary granule protein gene expression and investigated the signal transduction requirements for induction of secondary granule gene expression in 32D cells.
Materials and methods: Wild-type and variant 32D cells expressing normal or chimeric EPORs or receptors for granulocyte colony-stimulating factor (G-CSFRs) were stimulated with EPO or G-CSF and the expression of granulocyte-specific genes was analyzed by Northern blot analysis. To determine the signaling mechanisms required for secondary granule protein gene induction, the activation of STAT pathways following growth factor stimulation was studied by Western blot analysis.
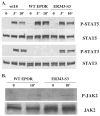
Results: We found that EPO treatment of 32D cells engineered to express EPOR did not result in induction of the secondary granule protein genes encoding lactoferrin and 24p3 lipocalin, the mouse homolog of human N-Gal, or the myeloid transcription factor C/EBPepsilon. Replacement of the intracellular domain of EPOR with the intracellular domain of G-CSFR in a chimeric receptor was associated with EPO-mediated induction of lactoferrin, 24p3 lipocalin, and C/EBPepsilon genes. We found that STAT3 phosphorylation was mediated by the intracellular domain of G-CSFR, but not EPOR. Replacement of one or two of the STAT5 binding sites in the intracytoplasmic domain of the EPOR with STAT3 binding sites resulted in EPO-mediated STAT3 activation and a marked increase in the expression of the 24p3 lipocalin gene. Knockdown of STAT3 protein levels with siRNA caused significant decrease in 24p3 lipocalin gene induction.
Conclusion: These results indicate that EPOR signaling cannot substitute for G-CSFR signaling to stimulate secondary granule protein gene expression in 32D cells. In addition, STAT3 is a critical mediator of 24p3 lipocalin gene expression in these cells.
Figures








Similar articles
-
The granulocyte colony-stimulating factor receptor supports erythroid differentiation in the absence of the erythropoietin receptor or Stat5.Br J Haematol. 2001 Feb;112(2):449-58. doi: 10.1046/j.1365-2141.2001.02591.x. Br J Haematol. 2001. PMID: 11167846
-
Erythropoietin mediates terminal granulocytic differentiation of committed myeloid cells with ectopic erythropoietin receptor expression.Eur J Haematol. 2001 Aug;67(2):77-87. Eur J Haematol. 2001. PMID: 11722594
-
The distal cytoplasmic domain of the erythropoietin receptor induces granulocytic differentiation in 32D cells.Blood. 1998 Aug 15;92(4):1219-24. Blood. 1998. PMID: 9694710
-
Control of myeloid differentiation and survival by Stats.Oncogene. 2000 May 15;19(21):2612-8. doi: 10.1038/sj.onc.1203477. Oncogene. 2000. PMID: 10851060 Review.
-
Stat3 and G-CSF-induced myeloid differentiation.Leuk Lymphoma. 1998 Aug;30(5-6):433-42. doi: 10.3109/10428199809057555. Leuk Lymphoma. 1998. PMID: 9711905 Review.
Cited by
-
HIF-1α downregulates miR-17/20a directly targeting p21 and STAT3: a role in myeloid leukemic cell differentiation.Cell Death Differ. 2013 Mar;20(3):408-18. doi: 10.1038/cdd.2012.130. Epub 2012 Oct 12. Cell Death Differ. 2013. PMID: 23059786 Free PMC article.
-
Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor.J Exp Med. 2009 May 11;206(5):1181-99. doi: 10.1084/jem.20082521. Epub 2009 May 4. J Exp Med. 2009. PMID: 19414555 Free PMC article.
-
A review of granulocyte colony-stimulating factor receptor signaling and regulation with implications for cancer.Front Oncol. 2022 Aug 11;12:932608. doi: 10.3389/fonc.2022.932608. eCollection 2022. Front Oncol. 2022. PMID: 36033452 Free PMC article. Review.
-
Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and 'emergency' hematopoiesis.Cytokine. 2008 Jun;42(3):277-88. doi: 10.1016/j.cyto.2008.03.002. Epub 2008 Apr 8. Cytokine. 2008. PMID: 18400509 Free PMC article. Review.
-
Innate immune regulation by STAT-mediated transcriptional mechanisms.Immunol Rev. 2014 Sep;261(1):84-101. doi: 10.1111/imr.12198. Immunol Rev. 2014. PMID: 25123278 Free PMC article. Review.
References
-
- Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. - PubMed
-
- Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor–deficient mice. Immunity. 1996;5:491–501. - PubMed
-
- Semerad CL, Poursine-Laurent J, Liu F, Link DC. A role for G-CSF receptor signaling in the regulation of hematopoietic cell function but not lineage commitment or differentiation. Immunity. 1999;11:153–161. - PubMed
-
- Mitsui T, Watanabe S, Taniguchi Y, et al. Impaired neutrophil maturation in truncated murine G-CSF receptor–transgenic mice. Blood. 2003;101:2990–2995. - PubMed
-
- D’Andrea AD. Hematopoietic growth factors and the regulation of differentiative decisions. Curr Opin Cell Biol. 1994;6:804–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

