Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities
- PMID: 15731030
- PMCID: PMC1064923
- DOI: 10.1128/IAI.73.3.1330-1342.2005
Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities
Abstract
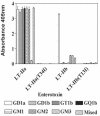
LT-IIa and LT-IIb, the type II heat-labile enterotoxins of Escherichia coli, are closely related in structure and function to cholera toxin and LT-I, the type I heat-labile enterotoxins of Vibrio cholerae and E. coli, respectively. Recent studies from our group demonstrated that LT-IIa and LT-IIb are potent systemic and mucosal adjuvants. To determine whether binding of LT-IIa and LT-IIb to their specific ganglioside receptors is essential for adjuvant activity, LT-IIa and LT-IIb enterotoxins were compared with their respective single-point substitution mutants which have no detectable binding activity for their major ganglioside receptors [e.g., LT-IIa(T34I) and LT-IIb(T13I)]. Both mutant enterotoxins exhibited an extremely low capacity for intoxicating mouse Y1 adrenal cells and for inducing production of cyclic AMP in a macrophage cell line. BALB/c female mice were immunized by the intranasal route with the surface adhesin protein AgI/II of Streptococcus mutans alone or in combination with LT-IIa, LT-IIa(T34I), LT-IIb, or LT-IIb(T13I). Both LT-IIa and LT-IIb potentiated strong mucosal and systemic immune responses against AgI/II. Of the two mutant enterotoxins, only LT-IIb(T13I) had the capacity to strongly potentiate mucosal anti-AgI/II and systemic anti-AgI/II antibody responses. Upon boosting with AgI/II, however, both LT-IIa(T34I) and LT-IIb(T13I) enhanced humoral memory responses to AgI/II. Flow cytometry demonstrated that LT-IIa(T34I) had no affinity for cervical lymph node lymphocytes. In contrast, LT-IIb(T13I) retained binding activity for T cells, B cells, and macrophages, indicating that this immunostimulatory mutant enterotoxin interacts with one or more unknown lymphoid cell receptors.
Figures


Similar articles
-
Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins.Expert Rev Vaccines. 2007 Oct;6(5):821-34. doi: 10.1586/14760584.6.5.821. Expert Rev Vaccines. 2007. PMID: 17931161 Free PMC article. Review.
-
Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants.Infect Immun. 2007 Feb;75(2):621-33. doi: 10.1128/IAI.01009-06. Epub 2006 Nov 21. Infect Immun. 2007. PMID: 17118982 Free PMC article.
-
Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb.Infect Immun. 2000 Jan;68(1):281-7. doi: 10.1128/IAI.68.1.281-287.2000. Infect Immun. 2000. PMID: 10603399 Free PMC article.
-
Binding to gangliosides containing N-acetylneuraminic acid is sufficient to mediate the immunomodulatory properties of the nontoxic mucosal adjuvant LT-IIb(T13I).Clin Vaccine Immunol. 2010 Jun;17(6):969-78. doi: 10.1128/CVI.00076-10. Epub 2010 Apr 14. Clin Vaccine Immunol. 2010. PMID: 20392887 Free PMC article.
-
The role of ADP-ribosylation and G(M1)-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin.Immunol Cell Biol. 1998 Jun;76(3):270-9. doi: 10.1046/j.1440-1711.1998.00745.x. Immunol Cell Biol. 1998. PMID: 9682971 Review.
Cited by
-
Mucosal pre-exposure to Th17-inducing adjuvants exacerbates pathology after influenza infection.Am J Pathol. 2014 Jan;184(1):55-63. doi: 10.1016/j.ajpath.2013.09.012. Epub 2013 Nov 1. Am J Pathol. 2014. PMID: 24183780 Free PMC article.
-
Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins.Expert Rev Vaccines. 2007 Oct;6(5):821-34. doi: 10.1586/14760584.6.5.821. Expert Rev Vaccines. 2007. PMID: 17931161 Free PMC article. Review.
-
LT-IIc, a new member of the type II heat-labile enterotoxin family, exhibits potent immunomodulatory properties that are different from those induced by LT-IIa or LT-IIb.Vaccine. 2011 Jan 17;29(4):721-7. doi: 10.1016/j.vaccine.2010.11.020. Epub 2010 Nov 21. Vaccine. 2011. PMID: 21095251 Free PMC article.
-
Structure-activity correlations of variant forms of the B pentamer of Escherichia coli type II heat-labile enterotoxin LT-IIb with Toll-like receptor 2 binding.Acta Crystallogr D Biol Crystallogr. 2012 Dec;68(Pt 12):1604-12. doi: 10.1107/S0907444912038917. Epub 2012 Nov 9. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 23151625 Free PMC article.
-
LT-IIc, a new member of the type II heat-labile enterotoxin family encoded by an Escherichia coli strain obtained from a nonmammalian host.Infect Immun. 2010 Nov;78(11):4705-13. doi: 10.1128/IAI.00730-10. Epub 2010 Aug 16. Infect Immun. 2010. PMID: 20713622 Free PMC article.
References
-
- Baudner, B. C., M. M. Giuliani, J. C. Verhoef, R. Rappuoli, H. E. Junginger, and G. D. Giudice. 2003. The concomitant use of the LTK63 mucosal adjuvant and of chitosan-based delivery system enhances the immunogenicity and efficacy of intranasally administered vaccines. Vaccine 21:3837-3844. - PubMed
-
- Brenner, S., S. Prosch, K. Schenke-Layland, U. Riese, U. Gausmann, and C. Platzer. 2003. cAMP-induced interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J. Biol. Chem. 278:5597-5604. - PubMed
-
- Cheng, E., L. Cardenas-Freytag, and J. D. Clements. 1999. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 18:38-49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

