Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling
- PMID: 15731043
- PMCID: PMC1064918
- DOI: 10.1128/IAI.73.3.1452-1465.2005
Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling
Abstract
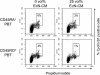
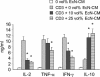
Although the probiotic Escherichia coli strain Nissle 1917 has been proven to be efficacious for the treatment of inflammatory bowel diseases, the underlying mechanisms of action still remain elusive. The aim of the present study was to analyze the effects of E. coli Nissle 1917 on cell cycling and apoptosis of peripheral blood and lamina propria T cells (PBT and LPT, respectively). Anti-CD3-stimulated PBT and LPT were treated with E. coli Nissle 1917-conditioned medium (E. coli Nissle 1917-CM) or heat-inactivated E. coli Nissle 1917. Cyclin B1, DNA content, and caspase 3 expression were measured by flow cytometry to assess cell cycle kinetics and apoptosis. Protein levels of several cell cycle and apoptosis modulators were determined by immunoblotting, and cytokine profiles were determined by cytometric bead array. E. coli Nissle 1917-CM inhibits cell cycling and expansion of peripheral blood but not mucosal T cells. Bacterial lipoproteins mimicked the effect of E. coli Nissle 1917-CM; in contrast, heat-inactivated E. coli Nissle 1917, lipopolysaccharide, or CpG DNA did not alter PBT cell cycling. E. coli Nissle 1917-CM decreased cyclin D2, B1, and retinoblastoma protein expression, contributing to the reduction of T-cell proliferation. E. coli Nissle 1917 significantly inhibited the expression of interleukin-2 (IL-2), tumor necrosis factor alpha, and gamma interferon but increased IL-10 production in PBT. Using Toll-like receptor 2 (TLR-2) knockout mice, we further demonstrate that the inhibition of PBT proliferation by E. coli Nissle 1917-CM is TLR-2 dependent. The differential reaction of circulating and tissue-bound T cells towards E. coli Nissle 1917 may explain the beneficial effect of E. coli Nissle 1917 in intestinal inflammation. E. coli Nissle 1917 may downregulate the expansion of newly recruited T cells into the mucosa and limit intestinal inflammation, while already activated tissue-bound T cells may eliminate deleterious antigens in order to maintain immunological homeostasis.
Figures




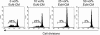

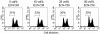

Similar articles
-
The probiotic Escherichia coli strain Nissle 1917 induces gammadelta T cell apoptosis via caspase- and FasL-dependent pathways.Int Immunol. 2008 Jul;20(7):829-40. doi: 10.1093/intimm/dxn041. Epub 2008 Apr 30. Int Immunol. 2008. PMID: 18448456
-
Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways.Infect Immun. 2006 Jul;74(7):4075-82. doi: 10.1128/IAI.01449-05. Infect Immun. 2006. PMID: 16790781 Free PMC article.
-
Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma.Arthritis Rheum. 2004 Dec;50(12):3856-65. doi: 10.1002/art.20678. Arthritis Rheum. 2004. PMID: 15593217
-
[Escherichia coli Nissle 1917 as safe vehicles for intestinal immune targeted therapy--a review].Wei Sheng Wu Xue Bao. 2013 Jun 4;53(6):538-44. Wei Sheng Wu Xue Bao. 2013. PMID: 24028055 Review. Chinese.
-
Clinical use of E. coli Nissle 1917 in inflammatory bowel disease.Inflamm Bowel Dis. 2008 Jul;14(7):1012-8. doi: 10.1002/ibd.20377. Inflamm Bowel Dis. 2008. PMID: 18240278 Review.
Cited by
-
Host-Microbiome Cross-talk in Oral Mucositis.J Dent Res. 2016 Jul;95(7):725-33. doi: 10.1177/0022034516641890. Epub 2016 Apr 6. J Dent Res. 2016. PMID: 27053118 Free PMC article. Review.
-
Effect of Probiotic E. coli Nissle 1917 Supplementation on the Growth Performance, Immune Responses, Intestinal Morphology, and Gut Microbes of Campylobacter jejuni Infected Chickens.Infect Immun. 2022 Oct 20;90(10):e0033722. doi: 10.1128/iai.00337-22. Epub 2022 Sep 22. Infect Immun. 2022. PMID: 36135600 Free PMC article.
-
Bacteriocinogeny in experimental pigs treated with indomethacin and Escherichia coli Nissle.World J Gastroenterol. 2011 Feb 7;17(5):609-17. doi: 10.3748/wjg.v17.i5.609. World J Gastroenterol. 2011. PMID: 21350709 Free PMC article.
-
Oral Immunization with Escherichia coli Nissle 1917 Expressing SARS-CoV-2 Spike Protein Induces Mucosal and Systemic Antibody Responses in Mice.Biomolecules. 2023 Mar 21;13(3):569. doi: 10.3390/biom13030569. Biomolecules. 2023. PMID: 36979504 Free PMC article.
-
The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics.Nat Rev Gastroenterol Hepatol. 2021 Sep;18(9):649-667. doi: 10.1038/s41575-021-00440-6. Epub 2021 May 4. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33948025 Free PMC article. Review.
References
-
- Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. - PubMed
-
- Allison, T. J., and D. N. Garboczi. 2002. Structure of γδ T cell receptors and their recognition of non-peptide antigens. Mol. Immunol. 38:1051-1061. - PubMed
-
- Allison, T. J., C. C. Winter, J. J. Fournie, M. Bonneville, and D. N. Garboczi. 2001. Structure of a human γδ T-cell antigen receptor. Nature 411:820-824. - PubMed
-
- Balomenos, D., and A. Martinez. 2000. Cell-cycle regulation in immunity, tolerance and autoimmunity. Immunol. Today 21:551-555. - PubMed
-
- Begg, A. C., N. J. McNally, D. C. Shrieve, and H. Karcher. 1985. A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry 6:620-626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

