Translocation and surface expression of lipidated serogroup B capsular Polysaccharide in Neisseria meningitidis
- PMID: 15731047
- PMCID: PMC1064937
- DOI: 10.1128/IAI.73.3.1491-1505.2005
Translocation and surface expression of lipidated serogroup B capsular Polysaccharide in Neisseria meningitidis
Abstract
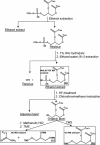
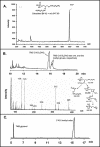
The capsule of N. meningitidis serogroup B, (alpha2-->8)-linked polysialic acid and the capsules of other meningococcal serogroups and of other gram-negative bacterial pathogens are anchored in the outer membrane through a 1,2-diacylglycerol moiety. Previous work on the meningococcal cps complex in Escherichia coli K-12 indicated that deletion of genes designated lipA and lipB caused intracellular accumulation of hyperelongated capsule polymers lacking the phospholipid substitution. To better understand the role of lip and lipB in capsule expression in a meningococcal background, the location, sequence, and relationship to related bacterial capsule genes were defined and specific mutations in lipA and lipB were generated in the serogroup B meningococcal strain NMB. The lipA and lipB genes are located on the 3' end of the ctr operon and are most likely transcribed independently. Inactivation of lipA, lipB, and both resulted in the same total levels of capsular polymer production as in the parental controls; however, these mutants were as sensitive as an unencapsulated mutant to killing by normal human serum. Immunogold electron microscopy and flow cytometric analyses revealed intracellular inclusions of capsular polymers in lipA, lipB, and lipA lipB mutants. Capsular polymers purified from lipA, lipB, and lipA lipB mutants were lipidated. The phospholipid anchor was shown by gas chromatography-mass spectroscopy analysis to be a phosphodiester-linked 1,2-dipalmitoyl (C16:0) glycerol moiety and was identical in structure to that found on the wild-type meningococcal capsule polymers. Thus, lipA and lipB do not encode proteins responsible for diacylglycerophosphatidic acid substitution of the meningococcal capsule polymer; rather, they are required for proper translocation and surface expression of the lipidated polymer.
Figures






Similar articles
-
The (alpha2-->8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum.Infect Immun. 1998 Dec;66(12):5939-47. doi: 10.1128/IAI.66.12.5939-5947.1998. Infect Immun. 1998. PMID: 9826376 Free PMC article.
-
Requirement of NMB0065 for connecting assembly and export of sialic acid capsular polysaccharides in Neisseria meningitidis.Microbes Infect. 2010 Jun;12(6):476-87. doi: 10.1016/j.micinf.2010.02.009. Epub 2010 Mar 7. Microbes Infect. 2010. PMID: 20215001 Free PMC article.
-
The Neisseria meningitidis serogroup A capsular polysaccharide O-3 and O-4 acetyltransferase.J Biol Chem. 2004 Oct 8;279(41):42765-73. doi: 10.1074/jbc.M313552200. Epub 2004 Aug 4. J Biol Chem. 2004. PMID: 15294916
-
Regulation of capsule in Neisseria meningitidis.Crit Rev Microbiol. 2016 Sep;42(5):759-72. doi: 10.3109/1040841X.2015.1022507. Epub 2015 Jun 19. Crit Rev Microbiol. 2016. PMID: 26089023 Free PMC article. Review.
-
Meningococcal vaccines.Pediatr Infect Dis J. 2004 Dec;23(12 Suppl):S285-92. Pediatr Infect Dis J. 2004. PMID: 15597071 Review.
Cited by
-
Neisseria meningitidis capsular polysaccharides induce inflammatory responses via TLR2 and TLR4-MD-2.J Leukoc Biol. 2011 Mar;89(3):469-80. doi: 10.1189/jlb.0610369. Epub 2010 Dec 29. J Leukoc Biol. 2011. PMID: 21191086 Free PMC article.
-
Description and nomenclature of Neisseria meningitidis capsule locus.Emerg Infect Dis. 2013 Apr;19(4):566-73. doi: 10.3201/eid1904.111799. Emerg Infect Dis. 2013. PMID: 23628376 Free PMC article.
-
The unique Legionella longbeachae capsule favors intracellular replication and immune evasion.PLoS Pathog. 2024 Sep 11;20(9):e1012534. doi: 10.1371/journal.ppat.1012534. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39259722 Free PMC article.
-
Viability of a capsule- and lipopolysaccharide-deficient mutant of Neisseria meningitidis.Infect Immun. 2005 Sep;73(9):6194-7. doi: 10.1128/IAI.73.9.6194-6197.2005. Infect Immun. 2005. PMID: 16113348 Free PMC article.
-
Identification of Campylobacter jejuni genes contributing to acid adaptation by transcriptional profiling and genome-wide mutagenesis.Appl Environ Microbiol. 2008 Mar;74(5):1598-612. doi: 10.1128/AEM.01508-07. Epub 2008 Jan 11. Appl Environ Microbiol. 2008. PMID: 18192408 Free PMC article.
References
-
- Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079-1093. - PubMed
-
- Bhattacharjee, A. K., H. J. Jennings, C. P. Kenny, A. Martin, and I. C. Smith. 1976. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can. J. Biochem. 54:1-8. - PubMed
-
- Bliss, J. M., and R. P. Silver. 1996. Coating the surface: a model for expression of capsular polysialic acid in Escherichia coli K1. Mol. Microbiol. 21:221-231. - PubMed
-
- Boyce, J. D., J. Y. Chung, and B. Adler. 2000. Genetic organisation of the capsule biosynthetic locus of Pasteurella multocida M1404 (B:2). Vet. Microbiol. 72:121-134. - PubMed
-
- Bronner, D., V. Sieberth, C. Pazzani, I. S. Roberts, G. J. Boulnois, B. Jann, and K. Jann. 1993. Expression of the capsular K5 polysaccharide of Escherichia coli: biochemical and electron microscopic analyses of mutants with defects in region 1 of the K5 gene cluster. J. Bacteriol. 175:5984-5992. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

