Effect of mutations in the human immunoglobulin A1 (IgA1) hinge on its susceptibility to cleavage by diverse bacterial IgA1 proteases
- PMID: 15731049
- PMCID: PMC1064975
- DOI: 10.1128/IAI.73.3.1515-1522.2005
Effect of mutations in the human immunoglobulin A1 (IgA1) hinge on its susceptibility to cleavage by diverse bacterial IgA1 proteases
Abstract
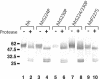
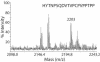
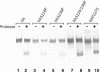
Components of the human immunoglobulin A1 (IgA1) hinge governing sensitivity to cleavage by bacterial IgA1 proteases were investigated. Recombinant antibodies with distinct hinge mutations were constructed from a hybrid comprised of human IgA2 bearing half of the human IgA1 hinge region. This hybrid antibody and all the mutant antibodies derived from it were resistant to cleavage by the IgA1 proteases from Streptococcus oralis and Streptococcus mitis biovar 1 strains but were cleaved to various degrees by those of Streptococcus pneumoniae, some Streptococcus sanguis strains, and the type 1 and 2 IgA1 proteases of Haemophilus influenzae, Neisseria meningitidis, and Neisseria gonorrhoeae. Remarkably, those proteases that cleave a Pro-Ser peptide bond in the wild-type IgA1 hinge were able to cleave mutant antibodies lacking a Pro-Ser peptide bond in the hinge, and those that cleave a Pro-Thr peptide bond in the wild-type IgA1 hinge were able to cleave mutant antibodies devoid of a Pro-Thr peptide bond in the hinge. Thus, the enzymes can cleave alternatives to their preferred postproline peptide bond when such a bond is unavailable. Peptide sequence analysis of a representative antibody digestion product confirmed this conclusion. The presence of a cleavable peptide bond near the CH2 end of the hinge appeared to result in greater cleavage than if the scissile bond was at the CH1 end of the hinge. Proline-to-serine substitution at residue 230 in a hinge containing potentially cleavable Pro-Ser and Pro-Thr peptide bonds increased the resistance of the antibody to cleavage by many IgA1 proteases.
Figures
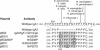


References
-
- Beck, S. C., and T. F. Meyer. 2000. IgA1 protease from Neisseria gonorrhoeae inhibits TNFα-mediated apoptosis of human monocytic cells. FEBS Lett. 472:287-292. - PubMed
-
- Blake, M., K. K. Holmes, and J. Swanson. 1979. Studies on gonococcus infection. XVII. IgA1-cleaving protease in vaginal washings from women with gonorrhea. J. Infect. Dis. 139:89-92. - PubMed
-
- Brooks, G. F., C. J. Lammel, M. S. Blake, B. Kusecek, and M. Achtman. 1992. Antibodies against IgA protease are stimulated both by clinical disease and asymptomatic carriage of serogroup A Neisseria meningitidis. J. Infect. Dis. 166:1316-1321. - PubMed
-
- Chintalacharuvu, K. R., P. D. Chuang, A. Dragoman, C. Z. Fernandez, J. Qiu, A. G. Plaut, K. R. Trinh, F. A. Gala, and S. L. Morrison. 2003. Cleavage of the human immunoglobulin A1 (IgA1) hinge region by IgA1 proteases requires structures in the Fc region of IgA. Infect. Immun. 71:2563-2570. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

