Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1
- PMID: 15731053
- PMCID: PMC1064940
- DOI: 10.1128/IAI.73.3.1553-1560.2005
Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1
Abstract
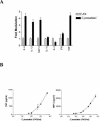
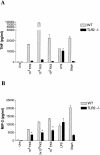
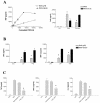
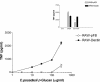
Coccidioides posadasii is a pathogenic fungus that causes endemic and epidemic coccidioidomycosis in the deserts of North, Central, and South America. How the innate immune system responds to the organism is not well understood. Here we show that elicited mouse peritoneal macrophages respond to spherules (the tissue form of the fungus) by producing proinflammatory cytokines as measured by quantitative PCR of cellular transcripts and by enzyme-linked immunosorbent assay (ELISA) assays for secreted protein. We examined the contribution of Toll-like receptors (TLR) and MyD88 in macrophage responses to formalin-killed spherules (FKS) by comparing cytokine responses of elicited macrophages from different knockout mice. FKS were added to elicited mouse peritoneal macrophages from wild-type, TLR2-/-, and MyD88-/- cells, and wild-type cells made more tumor necrosis factor alpha, MIP-2, and interleukin 6 than did the mutant macrophages. In contrast, the C3H/HeJ mice, which have a point mutation in TLR4, and TLR4-/- B6 mice exhibited no defect in cytokine production compared to the control mice. We also investigated the role of the macrophage beta-glucan receptor, Dectin-1. RAW 264.7 macrophages overexpressing Dectin-1 produced more cytokines in respond to FKS, live spherules, and purified beta-glucan than did control RAW cells. Blockage of Dectin-1 with antibodies inhibited cytokine production in elicited mouse peritoneal macrophages. Taken together, these results show that cytokine responses in mouse peritoneal macrophages to C. posadasii spherules are dependent on TLR2, MyD88, and Dectin-1.
Figures
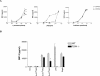
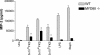
References
-
- Akira, S., M. Yamamoto, and K. Takeda. 2003. Role of adapters in Toll-like receptor signalling. Biochem. Soc. Trans. 31:637-642. - PubMed
-
- Ariizumi, K., G. L. Shen, S. Shikano, S. Xu, R. Ritter III, T. Kumamoto, D. Edelbaum, A. Morita, P. R. Bergstresser, and A. Takashima. 2000. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275:20157-20167. - PubMed
-
- Brown, G. D., and S. Gordon. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36-37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

