A live and inactivated Chlamydia trachomatis mouse pneumonitis strain induces the maturation of dendritic cells that are phenotypically and immunologically distinct
- PMID: 15731055
- PMCID: PMC1064943
- DOI: 10.1128/IAI.73.3.1568-1577.2005
A live and inactivated Chlamydia trachomatis mouse pneumonitis strain induces the maturation of dendritic cells that are phenotypically and immunologically distinct
Abstract
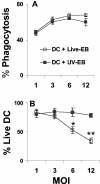
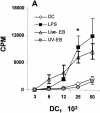
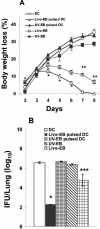
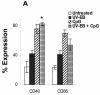
The intracellular bacterial pathogen Chlamydia trachomatis is a major cause of sexually transmitted disease worldwide. While protective immunity does appear to develop following natural chlamydial infection in humans, early vaccine trials using heat-killed C. trachomatis resulted in limited and transient protection with possible enhanced disease during follow-up. Thus, immunity following natural infection with live chlamydia may differ from immune responses induced by immunization with inactivated chlamydia. To study this differing immunology, we used murine bone marrow-derived dendritic cells (DC) to examine DC maturation and immune effector function induced by live and UV-irradiated C. trachomatis elementary bodies (live EBs and UV-EB, respectively). DC exposed to live EBs acquired a mature DC morphology; expressed high levels of major histocompatibility complex (MHC) class II, CD80, CD86, CD40, and ICAM-1; produced elevated amounts of interleukin-12 and tumor necrosis factor alpha; and were efficiently recognized by Chlamydia-specific CD4+ T cells. In contrast, UV-EB-pulsed DC expressed low levels of CD40 and CD86 but displayed high levels of MHC class II, ICAM-1, and CD80; secreted low levels of proinflammatory cytokines; and exhibited reduced recognition by Chlamydia-specific CD4+ T cells. Adoptive transfer of live EB-pulsed DC was more effective than that of UV-EB-pulsed DC at protecting mice against challenge with live C. trachomatis. The expression of DC maturation markers and immune protection induced by UV-EB could be significantly enhanced by costimulation of DC ex vivo with UV-EB and oligodeoxynucleotides containing cytosine phosphate guanosine; however, the level of protection was significantly less than that achieved by using DC pulsed ex vivo with viable EBs. Thus, exposure of DC to live EBs results in a mature DC phenotype which is able to promote protective immunity, while exposure to UV-EB generates a semimature DC phenotype with less protective potential. This result may explain in part the differences in protective immunity induced by natural infection and immunization with whole inactivated organisms and is relevant to rational chlamydia vaccine design strategies.
Figures




Similar articles
-
Genetic profiling of dendritic cells exposed to live- or ultraviolet-irradiated Chlamydia muridarum reveals marked differences in CXC chemokine profiles.Immunology. 2007 Feb;120(2):160-72. doi: 10.1111/j.1365-2567.2006.02488.x. Epub 2006 Oct 31. Immunology. 2007. PMID: 17073942 Free PMC article.
-
A peptide of Chlamydia trachomatis shown to be a primary T-cell epitope in vitro induces cell-mediated immunity in vivo.Immunology. 1995 May;85(1):8-15. Immunology. 1995. PMID: 7543450 Free PMC article.
-
Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae.J Exp Med. 1998 Sep 7;188(5):809-18. doi: 10.1084/jem.188.5.809. J Exp Med. 1998. PMID: 9730883 Free PMC article.
-
Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine.Nat Rev Immunol. 2005 Feb;5(2):149-61. doi: 10.1038/nri1551. Nat Rev Immunol. 2005. PMID: 15688042 Review.
-
The mucosal immune response to Chlamydia trachomatis infection of the reproductive tract in women.J Reprod Immunol. 2009 Dec;83(1-2):173-8. doi: 10.1016/j.jri.2009.07.013. Epub 2009 Nov 5. J Reprod Immunol. 2009. PMID: 19896206 Review.
Cited by
-
A multi-subunit Chlamydia vaccine inducing neutralizing antibodies and strong IFN-γ⁺ CMI responses protects against a genital infection in minipigs.Immunol Cell Biol. 2016 Feb;94(2):185-95. doi: 10.1038/icb.2015.79. Epub 2015 Aug 13. Immunol Cell Biol. 2016. PMID: 26268662 Free PMC article.
-
Irradiated whole cell Chlamydia vaccine confers significant protection in a murine genital tract challenge model.NPJ Vaccines. 2024 Nov 11;9(1):207. doi: 10.1038/s41541-024-00968-z. NPJ Vaccines. 2024. PMID: 39528548 Free PMC article.
-
Protective immunity against mouse upper genital tract pathology correlates with high IFNγ but low IL-17 T cell and anti-secretion protein antibody responses induced by replicating chlamydial organisms in the airway.Vaccine. 2012 Jan 5;30(2):475-85. doi: 10.1016/j.vaccine.2011.10.059. Epub 2011 Nov 10. Vaccine. 2012. PMID: 22079265 Free PMC article.
-
Immunization with dendritic cells pulsed ex vivo with recombinant chlamydial protease-like activity factor induces protective immunity against genital chlamydiamuridarum challenge.Front Immunol. 2011 Dec 8;2:73. doi: 10.3389/fimmu.2011.00073. eCollection 2011. Front Immunol. 2011. PMID: 22566862 Free PMC article.
-
Genetic profiling of dendritic cells exposed to live- or ultraviolet-irradiated Chlamydia muridarum reveals marked differences in CXC chemokine profiles.Immunology. 2007 Feb;120(2):160-72. doi: 10.1111/j.1365-2567.2006.02488.x. Epub 2006 Oct 31. Immunology. 2007. PMID: 17073942 Free PMC article.
References
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
-
- Birkelund, S., L. Bini, V. Pallini, M. Sanchez-Campillo, S. Liberatori, J. D. Clausen, S. Ostergaard, A. Holm, and G. Christiansen. 1997. Characterization of Chlamydia trachomatis l2-induced tyrosine-phosphorylated HeLa cell proteins by two-dimensional gel electrophoresis. Electrophoresis 18:563-567. - PubMed
-
- Brunham, R. C., J. Kimani, J. Bwayo, G. Maitha, I. Maclean, C. Yang, C. Shen, S. Roman, N. J. Nagelkerke, M. Cheang, and F. A. Plummer. 1996. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J. Infect. Dis. 173:950-956. - PubMed
-
- Brunham, R. C., D. J. Zhang, X. Yang, and G. M. McClarty. 2000. The potential for vaccine development against chlamydial infection and disease. J. Infect. Dis. 181(Suppl. 3):S538-S543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

