Rapid loss of motility of Helicobacter pylori in the gastric lumen in vivo
- PMID: 15731057
- PMCID: PMC1064960
- DOI: 10.1128/IAI.73.3.1584-1589.2005
Rapid loss of motility of Helicobacter pylori in the gastric lumen in vivo
Abstract
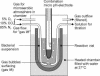
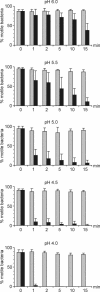
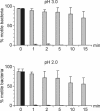
The human pathogen Helicobacter pylori has infected more than half of the world's population. Nevertheless, the first step of infection, the acute colonization of the gastric mucus, is poorly understood. For successful colonization, H. pylori must retain active motility in the gastric lumen until it reaches the safety of the mucus layer. To identify the factors determining the acute colonization, we inserted bacteria into the stomach of anesthetized Mongolian gerbils. We adjusted the gastric juice to defined pH values of between 2.0 and 6.0 by using an autotitrator. Despite the fact that Helicobacter spp. are known to survive low pH values for a certain time in vitro, the length of time that H. pylori persisted under the assay conditions within the gastric juice in vivo was remarkably shorter. In the anesthetized animal we found H. pylori to be irreversibly immotile in less than 1 min at lumen pH values of 2 and 3. At pH 4 motility was lost after 2 min. However, the period of motility increased to more than 15 min at pH 6. Blocking pepsins in the gastric lumen in vivo by using pepstatin significantly increased the period of motility. It was possible to simulate the rapid in vivo immotilization in vitro by adding pepsins. We conclude that pepsin limits the persistence of H. pylori in the gastric chymus to only a few minutes by rapidly inhibiting active motility. It is therefore likely that this short period of resistance in the gastric lumen is one of the most critical phases of Helicobacter infection.
Figures



References
-
- Agunod, M., N. Yamaguchi, R. Lopez, A. L. Luhby, and G. B. Glass. 1969. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am. J. Dig. Dis. 14:400-414. - PubMed
-
- Athauda, S. B., T. Kageyama, T. Takahashi, H. Inoue, M. Ichinose, M. Ukai, and K. Takahashi. 1995. Isolation and characterization of human gastric procathepsin E and cathepsin E. Adv. Exp. Med. Biol. 362:201-210. - PubMed
-
- Becker, T., and W. Rapp. 1979. Characterization of human pepsin I obtained from purified gastric pepsinogen I. Klin. Wochenschr. 57:711-718. - PubMed
-
- Becker, T., and W. Rapp. 1979. Characterization of human pepsin II obtained from purified gastric pepsinogen II. Klin. Wochenschr. 57:719-724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

