Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo
- PMID: 15731071
- PMCID: PMC1064930
- DOI: 10.1128/IAI.73.3.1706-1713.2005
Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo
Abstract
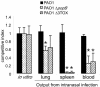
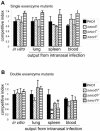
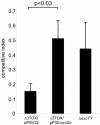
Pseudomonas aeruginosa uses a dedicated type III secretion system to deliver toxins directly into the cytoplasm of host cells. While progress has been made in elucidating the function of type III-secreted toxins in vitro, the in vivo functions of the type III-secreted exoenzymes are less well understood, particularly for the sequenced strain PAO1. Therefore, we have systematically deleted the genes for the three known type III effector molecules (exoS, exoT, and exoY) in P. aeruginosa PAO1 and assayed the effect of the deletions, both singly and in combination, on cytotoxicity in vitro and in vivo. We found that the type III secretion system acts differently on different cell types, causing an exoST-dependent rounding of a lung epithelial-like cell line in contrast to causing an exoSTY-independent but translocase (popB)-dependent lysis of a macrophage cell line. We utilized an in vivo competitive infection model to test each of our mutants, examining replication in the lung and spread to secondary sites such as the blood and spleen. Type III mutants inoculated intranasally exhibited only a minor defect in replication and survival in the lung, but popB and exoSTY triple mutants were profoundly defective in their ability to spread systemically. Intravenous injection of the mutants indicated that the type III secretion machinery is required for survival in the blood. Furthermore, our findings suggest that the effector-independent popB-dependent cytotoxicity that we and others have observed in vitro in macrophage cell lines may not be of great importance in vivo.
Figures

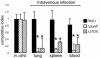
References
-
- Barbieri, J. T. 2000. Pseudomonas aeruginosa exoenzyme S, a bifunctional type-III secreted cytotoxin. Int. J. Med. Microbiol. 290:381-387. - PubMed
-
- Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. BioTechniques 29:948-950, 952. - PubMed
-
- Dacheux, D., J. Goure, J. Chabert, Y. Usson, and I. Attree. 2001. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol. Microbiol. 40:76-85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

