Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials
- PMID: 15731121
- PMCID: PMC552812
- DOI: 10.1136/bmj.38376.439653.D3
Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials
Abstract
Objective: To determine the efficacy and tolerability of topical pimecrolimus and tacrolimus compared with other treatments for atopic dermatitis.
Design: Systematic review and meta-analysis.
Data sources: Electronic searches of the Cochrane Library, Medline, and Embase.
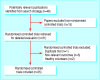
Study selection: Randomised controlled trials of topical pimecrolimus or tacrolimus reporting efficacy outcomes or tolerability.
Efficacy: investigators' global assessment of response; patients' global assessment of response; proportions of patients with flares of atopic dermatitis; and improvements in quality of life. Tolerability: overall rates of withdrawal; withdrawal due to adverse events; and proportions of patients with burning of the skin and skin infections.
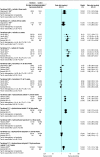
Data synthesis: 4186 of 6897 participants in 25 randomised controlled trials received pimecrolimus or tacrolimus. Both drugs were significantly more effective than a vehicle control. Tacrolimus 0.1% was as effective as potent topical corticosteroids at three weeks and more effective than combined treatment with hydrocortisone butyrate 0.1% (potent used on trunk) plus hydrocortisone acetate 1% (weak used on face) at 12 weeks (number needed to treat (NNT) = 6). Tacrolimus 0.1% was also more effective than hydrocortisone acetate 1% (NNT = 4). In comparison, tacrolimus 0.03% was more effective than hydrocortisone acetate 1% (NNT = 5) but less effective than hydrocortisone butyrate 0.1% (NNT = -8). Direct comparisons of tacrolimus 0.03% and tacrolimus 0.1% consistently favoured the higher strength formulation, but efficacy differed significantly between the two strengths only after 12 weeks' treatment (rate ratio 0.80, 95% confidence interval 0.65 to 0.99). Pimecrolimus was far less effective than betamethasone valerate 0.1% (NNT = -3 at three weeks). Pimecrolimus and tacrolimus caused significantly more skin burning than topical corticosteroids. Rates of skin infections in any of the comparisons did not differ.
Conclusions: Both topical pimecrolimus and topical tacrolimus are more effective than placebo treatments for atopic dermatitis, but in the absence of studies that show long term safety gains, any advantage over topical corticosteroids is unclear. Topical tacrolimus is similar to potent topical corticosteroids and may have a place for long term use in patients with resistant atopic dermatitis on sites where side effects from topical corticosteroids might develop quickly. In the absence of key comparisons with mild corticosteroids, the clinical need for topical pimecrolimus is unclear. The usefulness of either treatment in patients who have failed to respond adequately to topical corticosteroids is also unclear.
Figures

Comment in
-
Itching for a solution.BMJ. 2005 Mar 5;330(7490):522. doi: 10.1136/bmj.38391.604826.7C. Epub 2005 Feb 24. BMJ. 2005. PMID: 15731120 Free PMC article. No abstract available.
-
Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials.J Pediatr. 2005 Jul;147(1):126. doi: 10.1016/j.jpeds.2005.04.051. J Pediatr. 2005. PMID: 16027716 No abstract available.
-
Tacrolimus and pimecrolimus for atopic dermatitis: where do they fit in?Arch Dermatol. 2006 Sep;142(9):1203-5. doi: 10.1001/archderm.142.9.1203. Arch Dermatol. 2006. PMID: 16983008 No abstract available.
References
-
- Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J Allergy Clin Immunol 1999;103: 125-38. - PubMed
-
- Lewis-Jones MS, Finlay AY, Dykes PJ. The infants' dermatitis quality of life index. Br J Dermatol 2001;144: 104-10. - PubMed
-
- Kiebert G, Sorensen SV, Revicki D, Fagan SC, Doyle JJ, Cohen J, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol 2002;41: 151-8. - PubMed
-
- Herd RM. The morbidity and cost of atopic dermatitis. In: Williams HC, ed. Atopic dermatitis, Cambridge: Cambridge University Press, 2000.
-
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol 2001;144: 514-22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
