Protection of rhesus monkeys against infection with minimally pathogenic simian-human immunodeficiency virus: correlations with neutralizing antibodies and cytotoxic T cells
- PMID: 15731230
- PMCID: PMC1075715
- DOI: 10.1128/JVI.79.6.3358-3369.2005
Protection of rhesus monkeys against infection with minimally pathogenic simian-human immunodeficiency virus: correlations with neutralizing antibodies and cytotoxic T cells
Abstract
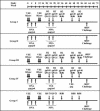
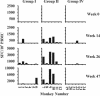
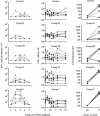
We studied the capacity of active immunization of rhesus monkeys with HIV-1 envelope protein (Env) to induce primary virus cross-reactive neutralizing antibodies to prevent infection following intravenous challenge with simian-human immunodeficiency virus (SHIV). Monkeys were immunized with the human immunodeficiency type 1 (HIV-1) strain R2 Env. Initially, the Env was expressed in vivo by an alphavirus replicon particle system, and then it was administered as soluble oligomeric gp140. Concurrently, groups of monkeys received expression vectors that encoded either simian immunodeficiency virus (SIV) gag/pol genes or no SIV genes in vivo to test the additional protective benefit of concurrent induction of virus-specific cell-mediated immune (CMI) responses. Groups of control monkeys received either the gag/pol regimen or sham immunizations. The antibodies induced by the Env immunization regimen neutralized diverse primary HIV-1 strains. Similarly, potent CMI responses were induced by the gag/pol regimen, as measured by gamma interferon enzyme-linked immunospot assays. Differences in the responses among groups of monkeys strongly suggested that there was interference between the Env and gag/pol immunization regimens. Complete protection of some of the monkeys against infection after intravenous challenge with the partially pathogenic SHIV(DH12R (Clone 7)) was associated independently with both neutralizing antibody and CMI responses. Protection was associated with SHIV(DH12 (Clone 7)) serum neutralizing antibody titers of > or =1:80 or with cellular immune responses corresponding to >2,000 spot forming cells per 10(6) peripheral blood mononuclear cells. Immunization was also associated with a reduction in the magnitude and duration of virus load. Induction of cross-reactive, primary HIV-1-neutralizing antibodies is feasible and, when potent, may result in complete protection against infection with a heterologous challenge virus strain.
Figures





Similar articles
-
Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys.J Virol. 2005 Oct;79(19):12321-31. doi: 10.1128/JVI.79.19.12321-12331.2005. J Virol. 2005. PMID: 16160159 Free PMC article.
-
Vaccination of rhesus macaques with recombinant Mycobacterium bovis bacillus Calmette-Guérin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif.J Virol. 2005 Feb;79(3):1452-62. doi: 10.1128/JVI.79.3.1452-1462.2005. J Virol. 2005. PMID: 15650171 Free PMC article.
-
Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection.J Virol. 2006 Sep;80(17):8745-62. doi: 10.1128/JVI.00956-06. J Virol. 2006. PMID: 16912322 Free PMC article.
-
Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1.Vaccine. 2002 May 6;20(15):1922-5. doi: 10.1016/s0264-410x(02)00068-3. Vaccine. 2002. PMID: 11983246 Review.
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
Cited by
-
Immunization of rabbits with highly purified, soluble, trimeric human immunodeficiency virus type 1 envelope glycoprotein induces a vigorous B cell response and broadly cross-reactive neutralization.PLoS One. 2014 May 20;9(5):e98060. doi: 10.1371/journal.pone.0098060. eCollection 2014. PLoS One. 2014. PMID: 24846288 Free PMC article.
-
Influence of Plasma Cell Niche Factors on the Recruitment and Maintenance of IRF4hi Plasma Cells and Plasmablasts in Vaccinated, Simian Immunodeficiency Virus-Infected Rhesus Macaques with Low and High Viremia.J Virol. 2017 Jan 31;91(4):e01727-16. doi: 10.1128/JVI.01727-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928009 Free PMC article.
-
Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge.Virology. 2008 Dec 20;382(2):217-25. doi: 10.1016/j.virol.2008.09.016. Epub 2008 Oct 22. Virology. 2008. PMID: 18947849 Free PMC article.
-
Neutralizing antibodies to HIV-1 induced by immunization.J Exp Med. 2013 Feb 11;210(2):209-23. doi: 10.1084/jem.20121827. J Exp Med. 2013. PMID: 23401570 Free PMC article. Review.
-
Biochemical and immunogenic characterization of soluble human immunodeficiency virus type 1 envelope glycoprotein trimers expressed by semliki forest virus.J Virol. 2005 Sep;79(17):10902-14. doi: 10.1128/JVI.79.17.10902-10914.2005. J Virol. 2005. PMID: 16103142 Free PMC article.
References
-
- Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. - PMC - PubMed
-
- Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. - PMC - PubMed
-
- Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. - PMC - PubMed
-
- Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. - PMC - PubMed
-
- Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

