3'-Terminal sequence in poliovirus negative-strand templates is the primary cis-acting element required for VPgpUpU-primed positive-strand initiation
- PMID: 15731251
- PMCID: PMC1075688
- DOI: 10.1128/JVI.79.6.3565-3577.2005
3'-Terminal sequence in poliovirus negative-strand templates is the primary cis-acting element required for VPgpUpU-primed positive-strand initiation
Abstract
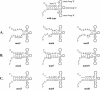
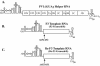
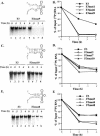
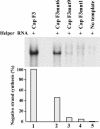
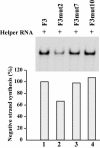
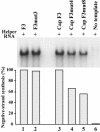
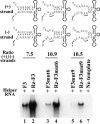
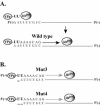
The 5' cloverleaf in poliovirus RNA has a direct role in regulating the stability, translation, and replication of viral RNA. In this study, we investigated the role of stem a in the 5' cloverleaf in regulating the stability and replication of poliovirus RNA in HeLa S10 translation-replication reactions. Our results showed that disrupting the duplex structure of stem a destabilized viral RNA and inhibited efficient negative-strand synthesis. Surprisingly, the duplex structure of stem a was not required for positive-strand synthesis. In contrast, altering the primary sequence at the 5'-terminal end of stem a had little or no effect on negative-strand synthesis but dramatically reduced positive-strand initiation and the formation of infectious virus. The inhibition of positive-strand synthesis observed in these reactions was most likely a consequence of nucleotide alterations in the conserved sequence at the 3' ends of negative-strand RNA templates. Previous studies suggested that VPgpUpU synthesized on the cre(2C) hairpin was required for positive-strand synthesis. Therefore, these results are consistent with a model in which preformed VPgpUpU serves as the primer for positive-strand initiation on the 3'AAUUUUGUC5' sequence at the 3' ends of negative-strand templates. Our results suggest that this sequence is the primary cis-acting element that is required for efficient VPgpUpU-primed positive-strand initiation.
Figures
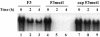
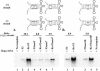
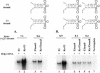

Similar articles
-
Poliovirus cre(2C)-dependent synthesis of VPgpUpU is required for positive- but not negative-strand RNA synthesis.J Virol. 2003 May;77(9):5136-44. doi: 10.1128/jvi.77.9.5136-5144.2003. J Virol. 2003. PMID: 12692216 Free PMC article.
-
Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis.J Virol. 2003 Apr;77(8):4739-50. doi: 10.1128/jvi.77.8.4739-4750.2003. J Virol. 2003. PMID: 12663781 Free PMC article.
-
5' cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis.EMBO J. 2001 Mar 15;20(6):1439-48. doi: 10.1093/emboj/20.6.1439. EMBO J. 2001. PMID: 11250909 Free PMC article.
-
Non-template functions of viral RNA in picornavirus replication.Curr Opin Virol. 2011 Nov;1(5):339-46. doi: 10.1016/j.coviro.2011.09.005. Curr Opin Virol. 2011. PMID: 22140418 Free PMC article. Review.
-
Molecular biology and cell-free synthesis of poliovirus.Biologicals. 1993 Dec;21(4):349-56. doi: 10.1006/biol.1993.1095. Biologicals. 1993. PMID: 8024750 Review.
Cited by
-
A 5'-proximal stem-loop structure of 5' untranslated region of porcine reproductive and respiratory syndrome virus genome is key for virus replication.Virol J. 2011 Apr 15;8:172. doi: 10.1186/1743-422X-8-172. Virol J. 2011. PMID: 21496223 Free PMC article.
-
5' terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart.Virology. 2008 Jun 5;375(2):480-91. doi: 10.1016/j.virol.2008.02.030. Epub 2008 Apr 1. Virology. 2008. PMID: 18378272 Free PMC article.
-
Protein-RNA tethering: the role of poly(C) binding protein 2 in poliovirus RNA replication.Virology. 2008 May 10;374(2):280-91. doi: 10.1016/j.virol.2007.12.039. Epub 2008 Feb 5. Virology. 2008. PMID: 18252259 Free PMC article.
-
5'-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA.J Virol. 2005 Jun;79(11):7024-41. doi: 10.1128/JVI.79.11.7024-7041.2005. J Virol. 2005. PMID: 15890942 Free PMC article.
-
Functional advantages of triplication of the 3B coding region of the FMDV genome.FASEB J. 2021 Feb;35(2):e21215. doi: 10.1096/fj.202001473RR. Epub 2020 Nov 23. FASEB J. 2021. PMID: 33230899 Free PMC article.
References
-
- Ambros, V., and D. Baltimore. 1978. Protein is linked to the 5′ end of poliovirus RNA by a phosphodiester linkage to tyrosine. J. Biol. Chem. 253:5263-5266. - PubMed
-
- Andino, R., G. E. Rieckhof, and D. Baltimore. 1990. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell 63:369-380. - PubMed
-
- Baltimore, D. 1969. The replication of poliovirus, p. 101-176. In The biochemistry of viruses. Marcel Dekker, New York, N.Y.
-
- Banerjee, R., and A. Dasgupta. 2001. Interaction of picornavirus 2C polypeptide with the viral negative-strand RNA. J. Gen. Virol. 82:2621-2627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

