Immunologic pressure within class I-restricted cognate human immunodeficiency virus epitopes during highly active antiretroviral therapy
- PMID: 15731259
- PMCID: PMC1075692
- DOI: 10.1128/JVI.79.6.3653-3663.2005
Immunologic pressure within class I-restricted cognate human immunodeficiency virus epitopes during highly active antiretroviral therapy
Abstract
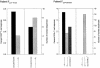
Cytotoxic T lymphocytes (CTL) and highly active antiretroviral therapy (HAART) are known to exert strong evolutionary pressures on the virus population during human immunodeficiency virus (HIV) infection. However, it is not known whether CTL responses continue to substantially affect viral evolution during treatment. To study the effect of immunologic pressure on viral sequences during HAART, we identified 10 targeted HIV-specific CD8+-T-cell epitopes in five treatment-naive patients, sequenced each epitope in plasma-derived viruses, and then identified evidence of immunologic pressure at these epitopes by comparing the frequency of viral variants in plasma to the frequency of the CD8+-T-cell response for each variant identified. For one of the five patients, evidence of viral evolution was found during therapy. The sequence of the CTL-targeted epitope changed from an apparent escape variant prior to the initiation of therapy, to the sequence that is best recognized by the CTL response after the initiation of therapy, and then finally to a new escape variant during continued therapy. These data show that CTL-mediated pressure can continue to affect viral evolution after the initiation of HAART, even when treatment drives the viral load below detectable levels, and suggest that antiretroviral therapy may preferentially inhibit those virus variants that escape the CTL response.
Figures





References
-
- Allen, T. M., M. Altfeld, X. G. Yu, K. M. O'Sullivan, M. Lichterfeld, S. Le Gall, M. John, B. R. Mothe, P. K. Lee, E. T. Kalife, D. E. Cohen, K. A. Freedberg, D. A. Strick, M. N. Johnston, A. Sette, E. S. Rosenberg, S. A. Mallal, P. J. Goulder, C. Brander, and B. D. Walker. 2004. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J. Virol. 78:7069-7078. - PMC - PubMed
-
- Alter, G., G. Hatzakis, C. M. Tsoukas, K. Pelley, D. Rouleau, R. LeBlanc, J. G. Baril, H. Dion, E. Lefebvre, R. Thomas, P. Cote, N. Lapointe, J. P. Routy, R. P. Sekaly, B. Conway, and N. F. Bernard. 2003. Longitudinal assessment of changes in HIV-specific effector activity in HIV-infected patients starting highly active antiretroviral therapy in primary infection. J. Immunol. 171:477-488. - PubMed
-
- Altfeld, M., M. M. Addo, R. Shankarappa, P. K. Lee, T. M. Allen, X. G. Yu, A. Rathod, J. Harlow, K. O'Sullivan, M. N. Johnston, P. J. Goulder, J. I. Mullins, E. S. Rosenberg, C. Brander, B. Korber, and B. D. Walker. 2003. Enhanced detection of human immunodeficiency virus type 1-specific T-cell responses to highly variable regions by using peptides based on autologous virus sequences. J. Virol. 77:7330-7340. - PMC - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

