BFRF1 of Epstein-Barr virus is essential for efficient primary viral envelopment and egress
- PMID: 15731264
- PMCID: PMC1075683
- DOI: 10.1128/JVI.79.6.3703-3712.2005
BFRF1 of Epstein-Barr virus is essential for efficient primary viral envelopment and egress
Abstract
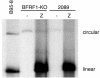
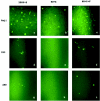
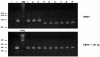
The molecular mechanisms that underlie maturation and egress of Epstein-Barr virus (EBV) virions are only partially characterized. We have recently shown that the BFRF1 gene, the EBV positional homolog of herpes simplex virus type 1 and pseudorabies virus UL34, is expressed early during EBV lytic replication and that it is found predominantly on the nuclear membrane (A. Farina, R. Santarelli, R. Gonnella, R. Bei, R. Muraro, G. Cardinali, S. Uccini, G. Ragona, L. Frati, A. Faggioni, and A. Angeloni, J. Virol. 74:3235-3244, 2000). These data suggest that the BFRF1 protein might be involved in viral primary envelopment. To precisely determine the function of this protein, we have constructed an EBV mutant devoid of the BFRF1 gene (BFRF1-KO). 293 cells carrying BFRF1-KO showed no differences in comparison with wild-type EBV in terms of DNA lytic replication or expression of late viral proteins upon induction of the lytic cycle. However, binding assays and infection experiments using cell lines or human cord blood lymphocytes showed a clear reduction in the viral mutant titers. Complementation experiments with BFRF1-KO and a BFRF1 expression vector restored viral titers to levels similar to those for the wild-type control, showing that the modifications that we introduced were limited to the BFRF1 gene. Electron microscopic observations showed that the reduction in viral titers was due to sequestration of EBV nucleocapsids in the nuclei of lytically induced cells. This suggests that BFRF1 is involved in transport of the maturing virion across the nuclear membrane. This hypothesis was further supported by the observation that BFRF1 is present in maturing intracellular virions but not in their extracellular counterparts. This implies that BFRF1 is a key protein for EBV maturation.
Figures





References
-
- Angeloni, A., A. Farina, G. Gentile, A. Capobianchi, P. Martino, V. Visco, R. Muraro, L. Frati, and A. Faggioni. 2001. Epstein-Barr virus and breast cancer: search for antibodies to the novel BFRF1 protein in sera of breast cancer patients. J. Natl. Cancer Inst. 93:560-561. - PubMed
-
- Ben-Bassat, H., N. Goldblum, S. Mitrani, T. Goldblum, J. M. Yoffey, M. M. Cohen, Z. Bentwich, B. Ramot, E. Klein, and G. Klein. 1977. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt like” malignant lymphoma (line D.G.-75). Int. J. Cancer 19:27-33. - PubMed
-
- Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

