The C-terminal half of TSG101 blocks Rous sarcoma virus budding and sequesters Gag into unique nonendosomal structures
- PMID: 15731271
- PMCID: PMC1075695
- DOI: 10.1128/JVI.79.6.3775-3786.2005
The C-terminal half of TSG101 blocks Rous sarcoma virus budding and sequesters Gag into unique nonendosomal structures
Abstract
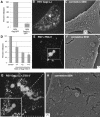
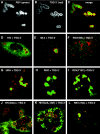
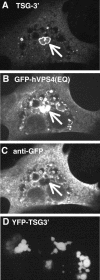
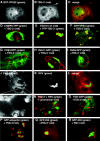
Retroviral late domains (L domains) are short amino acid sequences in the Gag protein that facilitate the process of budding. L domains act by recruiting the ESCRT complexes, which normally function in the formation of multivesicular bodies. The PTAP late domain of human immunodeficiency virus (HIV) is believed to specifically recruit this machinery by binding the ESCRT protein TSG101. It was recently demonstrated that expression of a C-terminal fragment of TSG101 (TSG-3') blocked the budding of both PTAP-dependent and PPPY-dependent retroviruses. We show here that TSG-3' expression leads to the formation of large spherical entities that we call TICS (TSG-3'-induced cellular structures) in the cytoplasm. Rous sarcoma virus (RSV) and murine leukemia virus (MLV) Gag proteins are selectively recruited to these structures, but HIV type 1 Gag is completely excluded. Experiments with various HIV and RSV vector constructs as well as HIV and RSV chimeras suggest that recruitment to the TICS is late domain independent and does not involve recognition of any single amino acid sequence. TICS appear to have no limiting membrane and do not colocalize with markers for any membranous cellular compartment. Wild-type TSG101 is also recruited to TICS, but most other ESCRT proteins are excluded. These structures are similar in nature to aggresomes, colocalize with the aggresome marker GFP-250, and are highly enriched in ubiquitin but in other ways do not fully meet the description of aggresomes. We conclude that the block to retroviral budding by TSG-3' may be the result of its sequestration of Gag, depletion of free TSG101, or depletion of free ubiquitin.
Figures


Similar articles
-
The functionally exchangeable L domains in RSV and HIV-1 Gag direct particle release through pathways linked by Tsg101.Traffic. 2005 Oct;6(10):880-94. doi: 10.1111/j.1600-0854.2005.00323.x. Traffic. 2005. PMID: 16138902 Free PMC article.
-
Defects in human immunodeficiency virus budding and endosomal sorting induced by TSG101 overexpression.J Virol. 2003 Jun;77(11):6507-19. doi: 10.1128/jvi.77.11.6507-6519.2003. J Virol. 2003. PMID: 12743307 Free PMC article.
-
Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function.Proc Natl Acad Sci U S A. 2002 Jan 22;99(2):955-60. doi: 10.1073/pnas.032511899. Proc Natl Acad Sci U S A. 2002. PMID: 11805336 Free PMC article.
-
[HIV budding and Tsg101].Uirusu. 2005 Dec;55(2):281-6. doi: 10.2222/jsv.55.281. Uirusu. 2005. PMID: 16557014 Review. Japanese.
-
Novel Tsg101 Binding Partners Regulate Viral L Domain Trafficking.Viruses. 2021 Jun 15;13(6):1147. doi: 10.3390/v13061147. Viruses. 2021. PMID: 34203832 Free PMC article. Review.
Cited by
-
The cellular factors Vps18 and Mon2 are required for efficient production of infectious HIV-1 particles.J Virol. 2011 Jun;85(11):5618-27. doi: 10.1128/JVI.00846-10. Epub 2011 Mar 30. J Virol. 2011. PMID: 21450827 Free PMC article.
-
The functionally exchangeable L domains in RSV and HIV-1 Gag direct particle release through pathways linked by Tsg101.Traffic. 2005 Oct;6(10):880-94. doi: 10.1111/j.1600-0854.2005.00323.x. Traffic. 2005. PMID: 16138902 Free PMC article.
-
Mechanisms of late restriction induced by an endogenous retrovirus.J Virol. 2007 Oct;81(20):11441-51. doi: 10.1128/JVI.01214-07. Epub 2007 Aug 15. J Virol. 2007. PMID: 17699582 Free PMC article.
-
Replacement of the hepatitis E virus ORF3 protein PxxP motif with heterologous late domain motifs affects virus release via interaction with TSG101.Virology. 2015 Dec;486:198-208. doi: 10.1016/j.virol.2015.09.012. Epub 2015 Oct 27. Virology. 2015. PMID: 26457367 Free PMC article.
-
Distinct Roles of Cellular ESCRT-I and ESCRT-III Proteins in Efficient Entry and Egress of Budded Virions of Autographa californica Multiple Nucleopolyhedrovirus.J Virol. 2017 Dec 14;92(1):e01636-17. doi: 10.1128/JVI.01636-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046462 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

