Effect of teosinte cytoplasmic genomes on maize phenotype
- PMID: 15731518
- PMCID: PMC1449101
- DOI: 10.1534/genetics.104.027300
Effect of teosinte cytoplasmic genomes on maize phenotype
Abstract
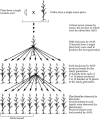
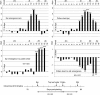
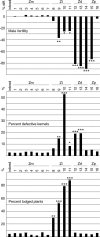
Determining the contribution of organelle genes to plant phenotype is hampered by several factors, including the paucity of variation in the plastid and mitochondrial genomes. To circumvent this problem, evolutionary divergence between maize (Zea mays ssp. mays) and the teosintes, its closest relatives, was utilized as a source of cytoplasmic genetic variation. Maize lines in which the maize organelle genomes were replaced through serial backcrossing by those representing the entire genus, yielding alloplasmic sublines, or cytolines were created. To avoid the confounding effects of segregating nuclear alleles, an inbred maize line was utilized. Cytolines with Z. mays teosinte cytoplasms were generally indistinguishable from maize. However, cytolines with cytoplasm from the more distantly related Z. luxurians, Z. diploperennis, or Z. perennis exhibited a plethora of differences in growth, development, morphology, and function. Significant differences were observed for 56 of the 58 characters studied. Each cytoline was significantly different from the inbred line for most characters. For a given character, variation was often greater among cytolines having cytoplasms from the same species than among those from different species. The characters differed largely independently of each other. These results suggest that the cytoplasm contributes significantly to a large proportion of plant traits and that many of the organelle genes are phenotypically important.
Figures

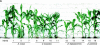
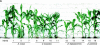





References
-
- Allen, J. O., 1992 Teosinte cytoplasmic genomes: interaction with maize nuclear genomes and molecular genetic characterization of the mitochondria. Ph.D. Thesis, University of Wisconsin, Madison, WI.
-
- Allen, J. O., G. K. Emenhiser and J. L. Kermicle, 1989. Miniature kernel and plant: interaction between teosinte cytoplasmic genomes and maize nuclear genomes. Maydica 34: 277–290.
-
- Beadle, G. W., 1980. The ancestry of corn. Sci. Am. 242: 112–119.
-
- Belliard, G., F. Vedel and G. Pelletier, 1979. Mitochondrial recombination in cytoplasmic hybrids of Nicotiana tabacum by protoplast fusion. Nature 281: 401–403.
-
- Benz, B. F., L. R. Sanchez-Velasquez and F. J. Santana Michel, 1990. Ecology and ethnobotany of Zea diploperennis: preliminary investigations. Maydica 35: 85–98.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

