Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity
- PMID: 15733303
- PMCID: PMC1571482
- DOI: 10.1111/j.1469-7580.2005.00378.x
Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity
Abstract
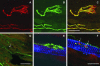
Palisade endings form a cuff of nerve terminals around the tip of muscle fibres. They are found only in extraocular muscles, but no definite evidence for their role in eye movements has been established. Palisade endings have been reported in all species so far investigated except the rat. In this study we demonstrate that antibodies against SNAP-25, the synaptosomal associated protein of 25 kDa, reliably visualize the complete motor, sensory and autonomic innervation of the extraocular muscles in human, monkey and rat. The SNAP-25 antibody can be combined with other immunofluorescence procedures, and is used here to study properties of palisade endings. With SNAP-25 immunolabelling putative palisade endings are identified in the rat for the first time. They are not well branched, but fulfil several criteria of palisade endings, being associated with non-twitch fibres as shown by double labelling with 'myosin heavy chain slow-twitch' antibodies. The putative palisade endings of the rat lack alpha-bungarotoxin binding, which implies that these synapses are sensory. If palisade endings are sensory then they could function as an eye muscle proprioceptor. They seem to be a general feature of all vertebrate eye muscles, unlike the other two extraocular proprioceptors, muscle spindles and Golgi tendon organs, the presence of which varies widely between species.
Figures


Similar articles
-
Proprioception and palisade endings in extraocular eye muscles.Ann N Y Acad Sci. 2005 Apr;1039:1-8. doi: 10.1196/annals.1325.001. Ann N Y Acad Sci. 2005. PMID: 15826956
-
Ultrastructural and molecular biologic comparison of classic proprioceptors and palisade endings in sheep extraocular muscles.Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5697-706. doi: 10.1167/iovs.09-3902. Epub 2009 Jun 24. Invest Ophthalmol Vis Sci. 2009. PMID: 19553627 Free PMC article.
-
Molecular characteristics suggest an effector function of palisade endings in extraocular muscles.Invest Ophthalmol Vis Sci. 2005 Jan;46(1):155-65. doi: 10.1167/iovs.04-1087. Invest Ophthalmol Vis Sci. 2005. PMID: 15623769
-
Proprioceptors in extraocular muscles.Exp Physiol. 2024 Jan;109(1):17-26. doi: 10.1113/EP090765. Epub 2023 Mar 3. Exp Physiol. 2024. PMID: 36869596 Free PMC article. Review.
-
Motor and sensory innervation of extraocular eye muscles.Ann N Y Acad Sci. 2003 Oct;1004:40-9. doi: 10.1111/j.1749-6632.2003.tb00240.x. Ann N Y Acad Sci. 2003. PMID: 14662446 Review.
Cited by
-
Is the central mesencephalic reticular formation a purely horizontal gaze center?Brain Struct Funct. 2022 Sep;227(7):2367-2393. doi: 10.1007/s00429-022-02532-8. Epub 2022 Jul 24. Brain Struct Funct. 2022. PMID: 35871423
-
Is there any sense in the Palisade endings of eye muscles?Ann N Y Acad Sci. 2011 Sep;1233:1-7. doi: 10.1111/j.1749-6632.2011.06169.x. Ann N Y Acad Sci. 2011. PMID: 21950969 Free PMC article.
-
Do palisade endings in extraocular muscles arise from neurons in the motor nuclei?Invest Ophthalmol Vis Sci. 2011 Apr 19;52(5):2510-9. doi: 10.1167/iovs.10-6008. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21228383 Free PMC article.
-
A central mesencephalic reticular formation projection to medial rectus motoneurons supplying singly and multiply innervated extraocular muscle fibers.J Comp Neurol. 2017 Jun 1;525(8):2000-2018. doi: 10.1002/cne.24187. Epub 2017 Mar 14. J Comp Neurol. 2017. PMID: 28177529 Free PMC article.
-
Evidence that the extraocular motor nuclei innervate monkey palisade endings.Neurosci Lett. 2011 Feb 4;489(2):89-93. doi: 10.1016/j.neulet.2010.11.072. Epub 2010 Dec 7. Neurosci Lett. 2011. PMID: 21138754 Free PMC article.
References
-
- Abuel-Atta AA, DeSantis M, Wong A. Encapsulated sensory receptors within intraorbital skeletal muscles of a camel. Anat. Rec. 1997;247:189–198. - PubMed
-
- Alvarado-Mallart RM, Pincon Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979;11:567–584. - PubMed
-
- Billig I, Buisseret-Delmas C, Buisseret P. Identification of nerve endings in cat extraocular muscles. Anat. Rec. 1997;248:566–575. - PubMed
-
- Blumer R, Lukas JR, Wasicky R, Mayr R. Presence and structure of innervated myotendinous cylinders in sheep extraocular muscle. Neurosci. Lett. 1998;248:49–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

