Diatom plastids possess a phosphoribulokinase with an altered regulation and no oxidative pentose phosphate pathway
- PMID: 15734914
- PMCID: PMC1065392
- DOI: 10.1104/pp.104.055285
Diatom plastids possess a phosphoribulokinase with an altered regulation and no oxidative pentose phosphate pathway
Abstract
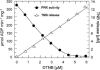
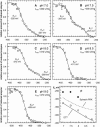
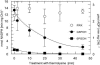
The chloroplast enzyme phosphoribulokinase (PRK; EC 2.7.1.19) is part of the Calvin cycle (reductive pentose phosphate pathway) responsible for CO(2) fixation in photosynthetic organisms. In green algae and vascular plants, this enzyme is light regulated via reversible reduction by reduced thioredoxin. We have sequenced and characterized the gene of the PRK from the marine diatom Odontella sinensis and found that the enzyme has the conserved cysteine residues necessary for thioredoxin-dependent regulation. Analysis of enzymatic activity of partially purified diatom enzyme and of purified protein obtained by native overexpression in Escherichia coli, however, revealed that under natural redox conditions the diatom enzyme is generally active. Treatment of the enzyme with strong oxidants results in inhibition of the enzyme, which is reversible by subsequent incubation with reducing agents. We determined the redox midpoint potentials of the regulatory cysteine in the PRK from O. sinensis in comparison to the respective spinach (Spinacia oleracea) enzyme and found a more positive redox potential for the diatom PRK, indicating that in vivo this enzyme might not be regulated by thioredoxin. We also demonstrate that in protease-treated diatom plastids, activities of enzymes of the oxidative pentose phosphate pathway are not detectable, thus reducing the need for a tight regulation of the Calvin cycle in diatoms. We discuss our results in the context of rearrangements of the subcellular compartmentation of metabolic pathways due to the peculiar evolution of diatoms by secondary endocytobiosis.
Figures


References
-
- Anderson LE (1973) Regulation of the pea leaf ribulose-5-phosphate kinase activity. Biochim Biophys Acta 321: 484–488 - PubMed
-
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, et al (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306: 79–86 - PubMed
-
- Badger MR, Hanson D, Price GD (2002) Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct Plant Biol 29: 161–173 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

