Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner
- PMID: 15738048
- PMCID: PMC2234012
- DOI: 10.1085/jgp.200409177
Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner
Abstract
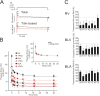
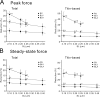
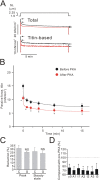
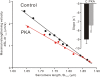
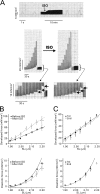
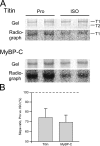
We investigated the effect of protein kinase A (PKA) on passive force in skinned cardiac tissues that express different isoforms of titin, i.e., stiff (N2B) and more compliant (N2BA) titins, at different levels. We used rat ventricular (RV), bovine left ventricular (BLV), and bovine left atrial (BLA) muscles (passive force: RV > BLV > BLA, with the ratio of N2B to N2BA titin, approximately 90:10, approximately 40:60, and approximately 10:90%, respectively) and found that N2B and N2BA isoforms can both be phosphorylated by PKA. Under the relaxed condition, sarcomere length was increased and then held constant for 30 min and the peak passive force, stress-relaxation, and steady-state passive force were determined. Following PKA treatment, passive force was significantly decreased in all muscle types with the effect greatest in RV, lowest in BLA, and intermediate in BLV. Fitting the stress-relaxation data to the sum of three exponential decay functions revealed that PKA blunts the magnitude of stress-relaxation and accelerates its time constants. To investigate whether or not PKA-induced decreases in passive force result from possible alteration of titin-thin filament interaction (e.g., via troponin I phosphorylation), we conducted the same experiments using RV preparations that had been treated with gelsolin to extract thin filaments. PKA decreased passive force in gelsolin-treated RV preparations with a magnitude similar to that observed in control preparations. PKA was also found to decrease restoring force in skinned ventricular myocytes of the rat that had been shortened to below the slack length. Finally, we investigated the effect of the beta-adrenergic receptor agonist isoprenaline on diastolic force in intact rat ventricular trabeculae. We found that isoprenaline phosphorylated titin and that it reduced diastolic force to a degree similar to that found in skinned RV preparations. Taken together, these results suggest that during beta-adrenergic stimulation, PKA increases ventricular compliance in a titin isoform-dependent manner.
Figures


Comment in
-
Modulation of cardiac function: titin springs into action.J Gen Physiol. 2005 Mar;125(3):249-52. doi: 10.1085/jgp.200509268. J Gen Physiol. 2005. PMID: 15738046 Free PMC article. Review. No abstract available.
References
-
- Bers, D.M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Second edition. Kluwer-Academic, Dordrecht, Netherlands. 427 pp.
-
- Bers, D.M. 2002. Cardiac excitation–contraction coupling. Nature. 415:198–205. - PubMed
-
- Cazorla, O., A. Freiburg, M. Helmes, T. Centner, M. McNabb, Y. Wu, K. Trombitas, S. Labeit, and H. Granzier. 2000. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ. Res. 86:59–67. - PubMed
-
- de Tombe, P.P., and H.E.D.J. ter Keurs. 1991. Lack of effect of isoproterenol on unloaded velocity of sarcomere shortening in rat cardiac trabeculae. Circ. Res. 68:382–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

