Crossing over is coupled to late meiotic prophase bivalent differentiation through asymmetric disassembly of the SC
- PMID: 15738262
- PMCID: PMC2171829
- DOI: 10.1083/jcb.200410144
Crossing over is coupled to late meiotic prophase bivalent differentiation through asymmetric disassembly of the SC
Abstract
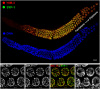
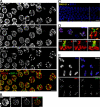
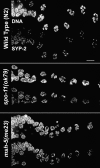
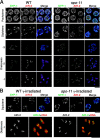
Homologous chromosome pairs (bivalents) undergo restructuring during meiotic prophase to convert a configuration that promotes crossover recombination into one that promotes bipolar spindle attachment and localized cohesion loss. We have imaged remodeling of meiotic chromosome structures after pachytene exit in Caenorhabditis elegans. Chromosome shortening during diplonema is accompanied by coiling of chromosome axes and highly asymmetric departure of synaptonemal complex (SC) central region proteins SYP-1 and SYP-2, which diminish over most of the length of each desynapsing bivalent while becoming concentrated on axis segments distal to the single emerging chiasma. This and other manifestations of asymmetry along chromosomes are lost in synapsis-proficient crossover-defective mutants, which often retain SYP-1,2 along the full lengths of coiled diplotene axes. Moreover, a gamma-irradiation treatment that restores crossovers in the spo-11 mutant also restores asymmetry of SYP-1 localization. We propose that crossovers or crossover precursors serve as symmetry-breaking events that promote differentiation of subregions of the bivalent by triggering asymmetric disassembly of the SC.
Figures

References
-
- Albertson, D.G., A.M. Rose, and A.M. Villeneuve. 1997. Chromosome organization, mitosis, and meiosis. C. elegans II. D.L. Riddle, T. Blumenthal, B.J. Meyer, and J.R. Priess, editors. Cold Spring Harbor Laboratory Press, Plainview, NY. 47–78. - PubMed
-
- Colaiácovo, M.P., A.J. MacQueen, E. Martinez-Perez, K. McDonald, A. Adamo, A. La Volpe, and A.M. Villeneuve. 2003. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell. 5:463–474. - PubMed
-
- Dernburg, A.F., K. McDonald, G. Moulder, R. Barstead, M. Dresser, and A.M. Villeneuve. 1998. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell. 94:387–398. - PubMed

