Regulation of the interaction between PIPKI gamma and talin by proline-directed protein kinases
- PMID: 15738269
- PMCID: PMC2171813
- DOI: 10.1083/jcb.200409028
Regulation of the interaction between PIPKI gamma and talin by proline-directed protein kinases
Abstract
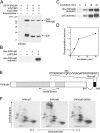
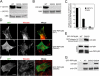
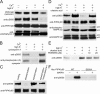
The interaction of talin with phosphatidylinositol(4) phosphate 5 kinase type I gamma (PIPKI gamma) regulates PI(4,5)P2 synthesis at synapses and at focal adhesions. Here, we show that phosphorylation of serine 650 (S650) within the talin-binding sequence of human PIPKI gamma blocks this interaction. At synapses, S650 is phosphorylated by p35/Cdk5 and mitogen-activated protein kinase at rest, and dephosphorylated by calcineurin upon stimulation. S650 is also a substrate for cyclin B1/Cdk1 and its phosphorylation in mitosis correlates with focal adhesion disassembly. Phosphorylation by Src of the tyrosine adjacent to S650 (Y649 in human PIPKI gamma) was shown to enhance PIPKI gamma targeting to focal adhesions (Ling, K., R.L. Doughman, V.V. Iyer, A.J. Firestone, S.F. Bairstow, D.F. Mosher, M.D. Schaller, and R.A. Anderson. 2003. J. Cell Biol. 163:1339-1349). We find that Y649 phosphorylation does not stimulate directly PIPKI gamma binding to talin, but may do so indirectly by inhibiting S650 phosphorylation. Conversely, S650 phosphorylation inhibits Y649 phosphorylation by Src. The opposite effects of the phosphorylation of Y649 and S650 likely play a critical role in regulating synaptic function as well as the balance between cell adhesion and cell motility.
Figures





References
-
- Ajiro, K., K. Yoda, K. Utsumi, and Y. Nishikawa. 1996. Alteration of cell cycle-dependent histone phosphorylations by okadaic acid. Induction of mitosis-specific H3 phosphorylation and chromatin condensation in mammalian interphase cells. J. Biol. Chem. 271:13197–13201. - PubMed
-
- Alessi, D.R., N. Gomez, G. Moorhead, T. Lewis, S.M. Keyse, and P. Cohen. 1995. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 5:283–295. - PubMed
-
- Barsukov, I.L., A. Prescot, N. Bate, B. Patel, D.N. Floyd, N. Bhanji, C.R. Bagshaw, K. Letinic, G. Di Paolo, P. De Camilli, et al. 2003. Phosphatidylinositol phosphate kinase type 1γ and β1-integrin cytoplasmic domain bind to the same region in the talin FERM domain. J. Biol. Chem. 278:31202–31209. - PubMed
-
- Bauerfeind, R., K. Takei, and P. De Camilli. 1997. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 272:30984–30992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

