Culturing at atmospheric oxygen levels impacts lymphocyte function
- PMID: 15738407
- PMCID: PMC553335
- DOI: 10.1073/pnas.0409910102
Culturing at atmospheric oxygen levels impacts lymphocyte function
Abstract
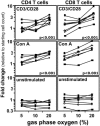
To determine whether culturing peripheral blood mononuclear cells at atmospheric oxygen levels skews responses in comparison with culturing lymphocytes at physiologic oxygen levels, we cultured peripheral blood mononuclear cells at 5%, 10%, and atmospheric (20%) gas-phase oxygen for 5 days. We found that incubator oxygen levels influenced lymphocyte proliferation stimulated by two commonly used stimuli: Con A and antibodies that crosslink surface CD3 and CD28 to mimic antigen presentation. In both cases, proliferation increased as gas-phase oxygen levels increased. In contrast, oxygen levels did not influence proliferation stimulated by phytohemagglutinin, another commonly used mitogen. Similarly, oxygen levels did not impact cell viability in unstimulated cultures. Thus, we conclude that the influence of oxygen levels on proliferation depends on the stimulus, and, most importantly from the standpoint of immune responses, culturing cells at atmospheric rather than physiologic oxygen levels results in significantly increased proliferation responses to the CD3/CD28 crosslinking, a proliferation stimulus commonly used to mimic T cell antigen receptor signaling.
Figures

References
-
- Steurer, J., Hoffmann, U., Dur, P., Russi, E. & Vetter, W. (1997) Respiration 64, 200-205. - PubMed
-
- Krieger, J. A., Landsiedel, J. C. & Lawrence, D. A. (1996) Int. J. Immunopharmacol. 18, 545-552. - PubMed
-
- Caldwell, C. C., Kojima, H., Lukashev, D., Armstrong, J., Farber, M., Apasov, S. G. & Sitkovsky, M. V. (2001) J. Immunol. 167, 6140-6149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

