Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis
- PMID: 15738413
- PMCID: PMC553290
- DOI: 10.1073/pnas.0404521102
Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis
Abstract
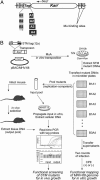
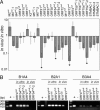
Gamma-herpesviruses, Epstein-Barr virus, and Kaposi's sarcoma-associated herpesvirus are important human pathogens, because they are involved in tumor development. Murine gamma-herpesvirus-68 (MHV-68 or gammaHV-68) has emerged as a small animal model system for the study of gamma-herpesvirus pathogenesis and host-virus interactions. To identify the genes required for viral replication in vitro and in vivo, we generated 1,152 mutants using signature-tagged transposon mutagenesis on an infectious bacterial artificial chromosome of MHV-68. Almost every ORF was mutated by random insertion. For each ORF, a mutant with an insertion proximal to the N terminus of each ORF was examined for the ability to grow in fibroblasts. Our results indicate that 41 genes are essential for in vitro growth, whereas 26 are nonessential and 6 attenuated. Replication-competent mutants were pooled to infect mice, which led to the discovery of ORF 54 being important for MHV-68 to replicate in the lung. This genetic analysis of a tumor-associated herpesvirus at the whole genome level validates signature-tagged transposon mutagenesis screening as an effective genetic system to identify important virulent genes in vivo and define interactions with the host immune system.
Figures


Similar articles
-
Identification of candidate gammaherpesvirus 68 genes required for virus replication by signature-tagged transposon mutagenesis.J Virol. 2004 Oct;78(19):10282-90. doi: 10.1128/JVI.78.19.10282-10290.2004. J Virol. 2004. PMID: 15367594 Free PMC article.
-
Regulation of the viral life cycle by murine gammaherpesvirus 68 microRNAs.Arch Virol. 2017 Mar;162(3):657-667. doi: 10.1007/s00705-016-3150-y. Epub 2016 Nov 11. Arch Virol. 2017. PMID: 27837274
-
Viral fitness of MHV-68 viruses harboring drug resistance mutations in the protein kinase or thymidine kinase.Antiviral Res. 2020 Oct;182:104901. doi: 10.1016/j.antiviral.2020.104901. Epub 2020 Aug 5. Antiviral Res. 2020. PMID: 32763314
-
Is murine gammaherpesvirus-68 (MHV-68) a suitable immunotoxicological model for examining immunomodulatory drug-associated viral recrudescence?J Immunotoxicol. 2015 Jan-Mar;12(1):1-15. doi: 10.3109/1547691X.2014.882996. Epub 2014 Feb 10. J Immunotoxicol. 2015. PMID: 24512328 Review.
-
Simian homologues of human herpesvirus 8.Philos Trans R Soc Lond B Biol Sci. 2001 Apr 29;356(1408):535-43. doi: 10.1098/rstb.2000.0782. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11313010 Free PMC article. Review.
Cited by
-
Interplay of Murine Gammaherpesvirus 68 with NF-kappaB Signaling of the Host.Front Microbiol. 2016 Aug 17;7:1202. doi: 10.3389/fmicb.2016.01202. eCollection 2016. Front Microbiol. 2016. PMID: 27582728 Free PMC article. Review.
-
Comprehensive Mutagenesis of Herpes Simplex Virus 1 Genome Identifies UL42 as an Inhibitor of Type I Interferon Induction.J Virol. 2019 Nov 13;93(23):e01446-19. doi: 10.1128/JVI.01446-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511375 Free PMC article.
-
High-resolution functional profiling of a gammaherpesvirus RTA locus in the context of the viral genome.J Virol. 2009 Feb;83(4):1811-22. doi: 10.1128/JVI.02302-08. Epub 2008 Dec 10. J Virol. 2009. PMID: 19073723 Free PMC article.
-
Persistent gammaherpesvirus replication and dynamic interaction with the host in vivo.J Virol. 2008 Dec;82(24):12498-509. doi: 10.1128/JVI.01152-08. Epub 2008 Oct 8. J Virol. 2008. PMID: 18842717 Free PMC article.
-
Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response.Cell Host Microbe. 2009 Feb 19;5(2):166-78. doi: 10.1016/j.chom.2008.12.013. Cell Host Microbe. 2009. PMID: 19218087 Free PMC article.
References
-
- Rickinson, A. B. & Kieff, E. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott William & Wilkins, Philadelphia), Vol. 2, pp. 2575-2627.
-
- Moore, P. S. & Chang, Y. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott William & Wilkins, Philadelphia), Vol. 2, pp. 2803-2833.
-
- Simas, J. P. & Efstathiou, S. (1998) Trends Microbiol. 6, 276-282. - PubMed
-
- Virgin, H. W. & Speck, S. H. (1999) Curr. Opin. Immunol. 11, 371-379. - PubMed
-
- Speck, S. H. & Virgin, H. W. (1999) Curr. Opin. Microbiol. 2, 403-409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

