Perinatal exposure to nicotine causes deficits associated with a loss of nicotinic receptor function
- PMID: 15738419
- PMCID: PMC552781
- DOI: 10.1073/pnas.0409782102
Perinatal exposure to nicotine causes deficits associated with a loss of nicotinic receptor function
Abstract
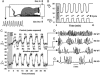
We investigated the role played by beta2-containing neuronal nicotinic receptors [nicotinic acetylcholine receptors (nAChRs)] in mediating nicotine's side effects in the fetus and newborn. Pregnant WT and mutant mice lacking the beta2 nAChR subunit were implanted with osmotic minipumps that delivered either water or a controlled dose of nicotine. Subsequently, we compared the development of the sympathoadrenal system and breathing and arousal reflexes of offspring shortly after birth, a period of increased vulnerability to nicotine exposure. Newborn WT pups exposed to nicotine exhibited all of the deficits associated with maternal tobacco and nicotine use, and linked to poor neonatal outcome: growth restriction, unstable breathing, and impaired arousal and catecholamine biosynthesis. Remarkably similar deficits were detected in pups lacking beta2-containing nAChRs. Loss-of-function of these nAChRs consequently reproduces with astonishing fidelity many of the abnormalities caused by perinatal nicotine exposure. We propose that the underlying mechanisms of nicotine's detrimental side effects on a range of crucial defensive reflexes involve loss of function of nAChR subtypes, possibly via activity-dependent desensitization.
Figures

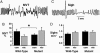

Similar articles
-
Mice lacking neuronal nicotinic acetylcholine receptor beta4-subunit and mice lacking both alpha5- and beta4-subunits are highly resistant to nicotine-induced seizures.Physiol Genomics. 2004 Apr 13;17(2):221-9. doi: 10.1152/physiolgenomics.00202.2003. Physiol Genomics. 2004. PMID: 14996991
-
Role of beta2-containing nicotinic acetylcholine receptors in auditory event-related potentials.Psychopharmacology (Berl). 2009 Mar;202(4):745-51. doi: 10.1007/s00213-008-1358-6. Epub 2008 Oct 18. Psychopharmacology (Berl). 2009. PMID: 18931833
-
beta 2 nicotinic acetylcholine receptor subunit modulates protective responses to stress: A receptor basis for sleep-disordered breathing after nicotine exposure.Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13272-7. doi: 10.1073/pnas.192463599. Epub 2002 Sep 12. Proc Natl Acad Sci U S A. 2002. PMID: 12228730 Free PMC article.
-
Neurobehavioral mechanisms of nicotine action: role in the initiation and maintenance of tobacco dependence.J Subst Abuse Treat. 1993 Mar-Apr;10(2):161-70. doi: 10.1016/0740-5472(93)90041-y. J Subst Abuse Treat. 1993. PMID: 8510190 Review.
-
Use of knock-out mice to determine the molecular basis for the actions of nicotine.Nicotine Tob Res. 1999;1 Suppl 2:S121-5; discussion S139-40. doi: 10.1080/14622299050011931. Nicotine Tob Res. 1999. PMID: 11768168 Review.
Cited by
-
Nicotine is neuroprotective to neonatal neurons of sympathetic ganglion in rat.Auton Neurosci. 2019 Jan;216:25-32. doi: 10.1016/j.autneu.2018.08.004. Epub 2018 Sep 4. Auton Neurosci. 2019. PMID: 30206032 Free PMC article.
-
Cholinergic modulation of appetite-related synapses in mouse lateral hypothalamic slice.J Neurosci. 2005 Nov 30;25(48):11133-44. doi: 10.1523/JNEUROSCI.3638-05.2005. J Neurosci. 2005. PMID: 16319313 Free PMC article.
-
Altered autonomic control in preterm newborns with impaired neurological outcomes.Clin Auton Res. 2015 Aug;25(4):233-42. doi: 10.1007/s10286-015-0298-6. Epub 2015 Aug 8. Clin Auton Res. 2015. PMID: 26253935
-
The Ferrier Lecture 1998. The molecular biology of consciousness investigated with genetically modified mice.Philos Trans R Soc Lond B Biol Sci. 2006 Dec 29;361(1476):2239-59. doi: 10.1098/rstb.2006.1832. Philos Trans R Soc Lond B Biol Sci. 2006. PMID: 17015398 Free PMC article.
-
Exposure to smoke during development: fetal programming of adult disease.Tob Induc Dis. 2006 Aug 15;3(2):5-16. doi: 10.1186/1617-9625-3-2-5. Tob Induc Dis. 2006. PMID: 19570293 Free PMC article.
References
-
- Walsh, R. S., Lowe, J. B. & Hopkins, P. J. (2001) Med. J. Aust. 175, 320-323. - PubMed
-
- NSW Mothers and Babies Report 1997 (1997) Part 3: Aboriginal and Torres Strait Islander Mothers and Babies, www.health.nsw.gov.au/public-health/mdc97/3_7.htm.
-
- Kleinman, J. C. (1988) Am. J. Epidemiol. 127, 274-282. - PubMed
-
- Mitchell, E. A. & Milerad, J. (1999) in WHO International Consultation on Environmental Tobacco Smoke and Child Health (W.H.O., Geneva), 115-129.
-
- Buka, S. L., Shenassa, E. D. & Niaura, R. (2003) Am. J. Psychiatry 160, 1978-1984. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

