Discovery of and mechanistic insight into a ligand-modulated palladium-catalyzed Wacker oxidation of styrenes using TBHP
- PMID: 15740083
- PMCID: PMC2720312
- DOI: 10.1021/ja043203m
Discovery of and mechanistic insight into a ligand-modulated palladium-catalyzed Wacker oxidation of styrenes using TBHP
Abstract
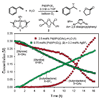
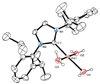
We describe the discovery of a new N-heterocyclic carbene-modulated Pd catalyst for the Wacker oxidation that does not require molecular oxygen but instead uses TBHP as a reagent in this oxidation. The catalyst activity and selectivity for the oxidation of styrene derivatives to methyl ketones are among the best reported. Preliminary mechanistic studies of the catalytic system were performed and show that the origin of the carbonyl oxygen is TBHP and that the hydrogens in the product all originate from the starting olefin.
Figures
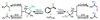
Similar articles
-
A general and efficient catalyst system for a Wacker-type oxidation using TBHP as the terminal oxidant: application to classically challenging substrates.J Am Chem Soc. 2009 May 6;131(17):6076-7. doi: 10.1021/ja901212h. J Am Chem Soc. 2009. PMID: 19364100 Free PMC article.
-
Direct conversion of allyl arenes to aryl ethylketones via a TBHP-mediated palladium-catalyzed tandem isomerization-Wacker oxidation of terminal alkenes.Org Biomol Chem. 2015 May 28;13(20):5613-6. doi: 10.1039/c5ob00586h. Epub 2015 Apr 17. Org Biomol Chem. 2015. PMID: 25884269
-
On the mechanism of the palladium-catalyzed tert-butylhydroperoxide-mediated Wacker-type oxidation of alkenes using quinoline-2-oxazoline ligands.J Am Chem Soc. 2011 Jun 1;133(21):8317-25. doi: 10.1021/ja2017043. Epub 2011 May 9. J Am Chem Soc. 2011. PMID: 21553838 Free PMC article.
-
Realization of Anti-Markovnikov Selectivity in Pd-Catalyzed Oxidative Acetalization and Wacker-Type Oxidation of Terminal Alkenes.Chem Rec. 2021 Dec;21(12):3458-3469. doi: 10.1002/tcr.202100090. Epub 2021 May 21. Chem Rec. 2021. PMID: 34021681 Review.
-
Sustainable Wacker-Type Oxidations.Angew Chem Int Ed Engl. 2022 Dec 12;61(50):e202211016. doi: 10.1002/anie.202211016. Epub 2022 Oct 26. Angew Chem Int Ed Engl. 2022. PMID: 36164675 Free PMC article. Review.
Cited by
-
Wacker Oxidation of Methylenecyclobutanes: Scope and Selectivity in an Unusual Setting.Angew Chem Int Ed Engl. 2023 Feb 6;62(7):e202215381. doi: 10.1002/anie.202215381. Epub 2023 Jan 12. Angew Chem Int Ed Engl. 2023. PMID: 36416612 Free PMC article.
-
A general and efficient catalyst system for a Wacker-type oxidation using TBHP as the terminal oxidant: application to classically challenging substrates.J Am Chem Soc. 2009 May 6;131(17):6076-7. doi: 10.1021/ja901212h. J Am Chem Soc. 2009. PMID: 19364100 Free PMC article.
-
Imparting catalyst control upon classical palladium-catalyzed alkenyl C-H bond functionalization reactions.Acc Chem Res. 2012 Jun 19;45(6):874-84. doi: 10.1021/ar200236v. Epub 2011 Nov 23. Acc Chem Res. 2012. PMID: 22111756 Free PMC article.
-
Construction of the tricyclic A-B-C core of the Veratrum alkaloids.J Org Chem. 2013 Sep 6;78(17):8437-41. doi: 10.1021/jo401158d. Epub 2013 Aug 6. J Org Chem. 2013. PMID: 23859604 Free PMC article.
-
(NHC)Pd(II) hydride-catalyzed dehydroaromatization by olefin chain-walking isomerization and transfer-dehydrogenation.Nat Commun. 2022 Sep 20;13(1):5507. doi: 10.1038/s41467-022-33163-6. Nat Commun. 2022. PMID: 36127352 Free PMC article.
References
-
-
Smidt J. Chem. Ind. 1962:54–62.Tsuji J. Synthesis. 1984;5:369–384.Acetophenone yield = 63%. (c) For a recent review, see: Takacs JM, Jiang X-T. Curr. Org. Chem. 2003;7:369–396.
-
-
- Nishimura T, Kakiuchi N, Onoue T, Ohe K, Uemura S. J. Chem. Soc., Perkin Trans. 1. 2000;12:1915–1918.
- ten Brink GJ, Arends IW, Papdogianakis G, Sheldon RA. Chem. Commun. 1998;21:2359–2360.
-
-
For an example using ionic liquids at high pressure, see:Namboodiri VV, Varma RS, Sahle-Demessie E, Pillai UR. Green Chem. 2002;4:170–173.Acetophenone yield = 79%. Using surfactants, see:Alandis N, Rico-Lattes I, Lattes A. New J. Chem. 1994;18:1147–1149.Yield = 83%. Using TBHP, see: Sommovigo M, Alper H. J. Mol. Catal. 1994;88:151–158.Yield = 30%. Using H2O2 and phase-transfer catalysis, see:Barak G, Sasson Y. J. Chem. Soc., Chem. Commun. 1987:1266–1267.Yield = 56%.
-
-
- Uozumi Y, Kato K, Hayashi T. J. Org. Chem. 1998;63:5071–5075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

