Ultrasound imaging versus morphopathology in cardiovascular diseases. Coronary collateral circulation and atherosclerotic plaque
- PMID: 15740620
- PMCID: PMC554094
- DOI: 10.1186/1476-7120-3-6
Ultrasound imaging versus morphopathology in cardiovascular diseases. Coronary collateral circulation and atherosclerotic plaque
Abstract
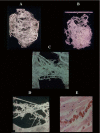
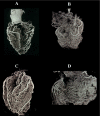
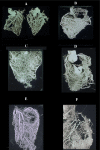
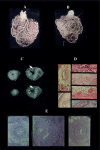
This review article is aimed at comparing the results of histopathological and clinical imaging studies to assess coronary collateral circulation in humans. The role of collaterals, as emerging from morphological studies in both normal and atherosclerotic coronary vessels, is described; in addition, present role and future perpectives of echocardiographic techniques in assessing collateral circulation are briefly summarized.
Figures



References
-
- Helfant RH, Kemp HG, Gorlin R. The interrelation between extent of coronary artery disease, presence of collaterals, ventriculographic abnormalities and hemodynamics. Am J Cardiol. 1970;25:102. doi: 10.1016/0002-9149(70)90875-1. - DOI
-
- Baroldi G, Mantero O, Scomazzoni G. The collaterals of the coronary arteries in normal and pathologic hearts. Circ Res. 1956;4:223. - PubMed
-
- Baroldi G, Scomazzoni G. American Registry of Pathology, AFIP. Washington D.C., U.S. Government Printing Office; 1967. Coronary Circulation in the Normal and Pathological Heart.
-
- DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, Lang HT. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303:897. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

