An efficient system to establish multiple embryonic stem cell lines carrying an inducible expression unit
- PMID: 15741176
- PMCID: PMC552969
- DOI: 10.1093/nar/gni043
An efficient system to establish multiple embryonic stem cell lines carrying an inducible expression unit
Abstract
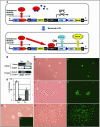
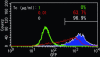
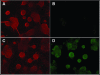
The growing use of mouse embryonic stem (ES) cells in research emphasizes their importance in studies of molecular mechanisms that maintain pluripotency and direct cellular differentiation. Although systems for regulatable transgene expression are essential for fine analysis of cellular processes at the molecular level, a strategy for the establishment of multiple ES cell lines carrying any of these systems has not yet been described. Here, we report our development of the ROSA-TET system, an effective system for the establishment of multiple ES cell lines carrying a tetracycline (Tc)-regulatable transgene at the Gt (ROSA)26asSor (ROSA26) locus. This system contains a knock-in step of a construct carrying both loxP and its mutant sequences into the ROSA26 locus, followed by a subsequent exchange step that introduces a cDNA to be Tc-regulated to the locus using the recombinase-mediated cassette exchange reaction. Both steps are demonstrated to give desired clones with high efficiency, suggesting that this system can be introduced readily into any ES cell lines, leading to the simultaneous establishment of multiple cell lines carrying different Tc-regulated cDNAs. We believe that use of this system will strongly accelerate molecular biological research using ES cells.
Figures

Similar articles
-
Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells.Genesis. 2006 Jan;44(1):23-8. doi: 10.1002/gene.20180. Genesis. 2006. PMID: 16400644
-
Constitutive Gs activation using a single-construct tetracycline-inducible expression system in embryonic stem cells and mice.Stem Cell Res Ther. 2011 Mar 4;2(2):11. doi: 10.1186/scrt52. Stem Cell Res Ther. 2011. PMID: 21375737 Free PMC article.
-
Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells.Stem Cells. 2004;22(2):225-35. doi: 10.1634/stemcells.22-2-225. Stem Cells. 2004. PMID: 14990861
-
Embryonic stem cell lines of nonhuman primates.ScientificWorldJournal. 2002 Jun 26;2:1762-73. doi: 10.1100/tsw.2002.829. ScientificWorldJournal. 2002. PMID: 12806169 Free PMC article. Review.
-
Human embryonic stem cells.J Cell Sci. 2000 Jan;113 ( Pt 1):5-10. doi: 10.1242/jcs.113.1.5. J Cell Sci. 2000. PMID: 10591620 Review.
Cited by
-
Determination of local chromatin composition by CasID.Nucleus. 2016 Sep 2;7(5):476-484. doi: 10.1080/19491034.2016.1239000. Epub 2016 Sep 27. Nucleus. 2016. PMID: 27676121 Free PMC article.
-
Extra-embryonic endoderm cells derived from ES cells induced by GATA factors acquire the character of XEN cells.BMC Dev Biol. 2007 Jul 3;7:80. doi: 10.1186/1471-213X-7-80. BMC Dev Biol. 2007. PMID: 17605826 Free PMC article.
-
Sox7 is dispensable for primitive endoderm differentiation from mouse ES cells.BMC Dev Biol. 2015 Oct 16;15:37. doi: 10.1186/s12861-015-0079-4. BMC Dev Biol. 2015. PMID: 26475439 Free PMC article.
-
Pax2 overexpression in embryoid bodies induces upregulation of integrin alpha8 and aquaporin-1.In Vitro Cell Dev Biol Anim. 2009 Jan-Feb;45(1-2):62-8. doi: 10.1007/s11626-008-9151-8. Epub 2008 Nov 27. In Vitro Cell Dev Biol Anim. 2009. PMID: 19037705
-
A high throughput embryonic stem cell screen identifies Oct-2 as a bifunctional regulator of neuronal differentiation.Genes Dev. 2009 Mar 1;23(5):575-88. doi: 10.1101/gad.1772509. Genes Dev. 2009. PMID: 19270158 Free PMC article.
References
-
- Lewandoski M. Conditional control of gene expression in the mouse. Nature Rev. Genet. 2001;2:743–755. - PubMed
-
- Gossen M., Bujard H. Studying gene function in eukaryotes by conditional gene inactivation. Annu. Rev. Genet. 2002;36:153–173. - PubMed
-
- Gossen M., Freundlieb S., Bender G., Muller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

