Higher-order nuclear organization in growth arrest of human mammary epithelial cells: a novel role for telomere-associated protein TIN2
- PMID: 15741234
- PMCID: PMC2933191
- DOI: 10.1242/jcs.01709
Higher-order nuclear organization in growth arrest of human mammary epithelial cells: a novel role for telomere-associated protein TIN2
Abstract
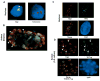
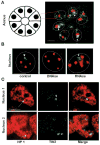
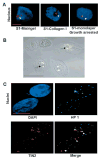
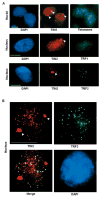
Nuclear organization, such as the formation of specific nuclear subdomains, is generally thought to be involved in the control of cellular phenotype; however, there are relatively few specific examples of how mammalian nuclei organize during radical changes in phenotype, such as those occurring during differentiation and growth arrest. Using human mammary epithelial cells in which growth arrest is essential for morphological differentiation, we show that the arrest of cell proliferation is accompanied by a reorganization of the telomere-associated protein, TIN2, into one to three large nuclear subdomains. The large TIN2 domains do not contain telomeres and occur concomitant with the continued presence of TIN2 at telomeres. The TIN2 domains were sensitive to DNase, but not RNase, occurred frequently, but not exclusively near nucleoli, and overlapped often with dense domains containing heterochromatin protein 1gamma. Expression of truncated forms of TIN2 simultaneously prevented the formation of TIN2 domains and relaxed the stringent morphogenesis-induced growth arrest in human mammary epithelial cells. Here we show that a novel extra-telomeric organization of TIN2 is associated with the control of cell proliferation and identify TIN2 as an important regulator of mammary epithelial differentiation.
Figures
Similar articles
-
A novel form of the telomere-associated protein TIN2 localizes to the nuclear matrix.Cell Cycle. 2009 Mar 15;8(6):931-9. doi: 10.4161/cc.8.6.7941. Epub 2009 Mar 26. Cell Cycle. 2009. PMID: 19229133 Free PMC article.
-
TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres.J Biol Chem. 2004 Nov 5;279(45):47264-71. doi: 10.1074/jbc.M409047200. Epub 2004 Aug 16. J Biol Chem. 2004. PMID: 15316005
-
Telomere dysfunction and cell survival: roles for distinct TIN2-containing complexes.J Cell Biol. 2008 May 5;181(3):447-60. doi: 10.1083/jcb.200710028. Epub 2008 Apr 28. J Cell Biol. 2008. PMID: 18443218 Free PMC article.
-
Shelterin: the protein complex that shapes and safeguards human telomeres.Genes Dev. 2005 Sep 15;19(18):2100-10. doi: 10.1101/gad.1346005. Genes Dev. 2005. PMID: 16166375 Review.
-
Inside the mammalian telomere interactome: regulation and regulatory activities of telomeres.Crit Rev Eukaryot Gene Expr. 2006;16(2):103-18. doi: 10.1615/critreveukargeneexpr.v16.i2.10. Crit Rev Eukaryot Gene Expr. 2006. PMID: 16749892 Review.
Cited by
-
NuMA influences higher order chromatin organization in human mammary epithelium.Mol Biol Cell. 2007 Feb;18(2):348-61. doi: 10.1091/mbc.e06-06-0551. Epub 2006 Nov 15. Mol Biol Cell. 2007. PMID: 17108325 Free PMC article.
-
Telomere dynamics: the means to an end.Cell Prolif. 2007 Aug;40(4):462-74. doi: 10.1111/j.1365-2184.2007.00452.x. Cell Prolif. 2007. PMID: 17635515 Free PMC article. Review.
-
Three-dimensional culture of human breast epithelial cells: the how and the why.Methods Mol Biol. 2013;945:193-219. doi: 10.1007/978-1-62703-125-7_13. Methods Mol Biol. 2013. PMID: 23097109 Free PMC article.
-
Induction of alternative lengthening of telomeres-associated PML bodies by p53/p21 requires HP1 proteins.J Cell Biol. 2009 Jun 1;185(5):797-810. doi: 10.1083/jcb.200810084. Epub 2009 May 25. J Cell Biol. 2009. PMID: 19468068 Free PMC article.
-
Statistical analysis of 3D images detects regular spatial distributions of centromeres and chromocenters in animal and plant nuclei.PLoS Comput Biol. 2010 Jul 8;6(7):e1000853. doi: 10.1371/journal.pcbi.1000853. PLoS Comput Biol. 2010. PMID: 20628576 Free PMC article.
References
-
- Bilaud T, Brun C, Ancelin K, Koering CE, Laroche T, Gilson E. Telomeric localization of TRF2, a novel human telobox protein. Nat Genet. 1997;17:236–239. - PubMed
-
- Braun N, Papadopoulos T, Muller-Hermelink HK. Cell cycle dependent distribution of the proliferation-associated Ki-67 antigen in human embryonic lung cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56:25–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

