Helminth regulation of host IL-4Ralpha/Stat6 signaling: mechanism underlying NOS-2 inhibition by Trichinella spiralis
- PMID: 15741272
- PMCID: PMC554809
- DOI: 10.1073/pnas.0409461102
Helminth regulation of host IL-4Ralpha/Stat6 signaling: mechanism underlying NOS-2 inhibition by Trichinella spiralis
Abstract
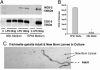
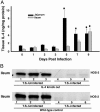
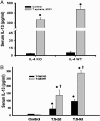
Gastrointestinal nematode infection is known to alter host T cell activation and has been used to study immune and inflammatory reactions in which nitric oxide (NO) is a versatile player. We previously demonstrated that Trichinella spiralis infection inhibits host inducible NO synthase (NOS-2) expression. We now demonstrate that (i) an IL-4 receptor alpha-subunit (IL-4Ralpha)/Stat6-dependent but T cell-independent pathway is the key for the nematode-induced host NOS-2 inhibition; (ii) endogenous IL-4 and IL-13, the only known IL-4Ralpha ligands, are not required for activating the pathway; and (iii) treatment of RAW264.7 cells with parasite-cultured medium inhibits NOS-2 expression but not cyclooxygenase 2 expression. We propose that a yet-unidentified substance is released by the nematode during the host-parasite interaction.
Figures



Similar articles
-
Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism.J Immunol. 2000 Feb 15;164(4):2046-52. doi: 10.4049/jimmunol.164.4.2046. J Immunol. 2000. PMID: 10657657
-
Regulation of recombinant Trichinella spiralis 53-kDa protein (rTsP53) on alternatively activated macrophages via STAT6 but not IL-4Rα in vitro.Cell Immunol. 2014 Mar-Apr;288(1-2):1-7. doi: 10.1016/j.cellimm.2014.01.010. Epub 2014 Feb 6. Cell Immunol. 2014. PMID: 24534206
-
Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells.Am J Physiol Gastrointest Liver Physiol. 2002 Feb;282(2):G226-32. doi: 10.1152/ajpgi.2002.282.2.G226. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 11804843
-
Interleukin-4 receptor signaling pathways in asthma pathogenesis.Trends Mol Med. 2004 Oct;10(10):493-9. doi: 10.1016/j.molmed.2004.08.004. Trends Mol Med. 2004. PMID: 15464449 Review.
-
Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites.Immunol Rev. 2004 Oct;201:139-55. doi: 10.1111/j.0105-2896.2004.00192.x. Immunol Rev. 2004. PMID: 15361238 Review.
Cited by
-
Kinetics Evaluation of IgM and IgG Levels in the Mice Infected with Trichinella spiralis Experimentally Using ES Antigens from Different Developmental Stages of the Parasite.Iran J Parasitol. 2019 Apr-Jun;14(2):223-230. Iran J Parasitol. 2019. PMID: 31543910 Free PMC article.
-
Trichinella-induced immunomodulation: Another tale of helminth success.Food Waterborne Parasitol. 2022 May 16;27:e00164. doi: 10.1016/j.fawpar.2022.e00164. eCollection 2022 Jun. Food Waterborne Parasitol. 2022. PMID: 35615625 Free PMC article.
-
Eosinophil-Mediated Immune Control of Adult Filarial Nematode Infection Can Proceed in the Absence of IL-4 Receptor Signaling.J Immunol. 2020 Aug 1;205(3):731-740. doi: 10.4049/jimmunol.1901244. Epub 2020 Jun 22. J Immunol. 2020. PMID: 32571840 Free PMC article.
-
Mesenchymal stem cells: a double-edged sword in regulating immune responses.Cell Death Differ. 2012 Sep;19(9):1505-13. doi: 10.1038/cdd.2012.26. Epub 2012 Mar 16. Cell Death Differ. 2012. PMID: 22421969 Free PMC article.
-
Helminth infection and type 1 diabetes.Rev Diabet Stud. 2012 Winter;9(4):272-86. doi: 10.1900/RDS.2012.9.272. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804266 Free PMC article. Review.
References
-
- Finkelman, F. D., Pearce, E. J., Urban, J. F., Jr., & Sher, A. (1991) Immunol. Today 12, A62–A66. - PubMed
-
- Garside, P., Kennedy, M. W., Wakelin, D. & Lawrence, C. E. (2000) Parasite Immunol. 22, 605–612. - PubMed
-
- Finkelman, F. D. & Urban, J. F., Jr. (2001) J. Allergy Clin. Immunol. 107, 772–780. - PubMed
-
- Yazdanbakhsh, M., Kremsner, P. G. & van Ree, R. (2002) Science 296, 490–494. - PubMed
-
- Bian, K. & Murad, F. (2003) Frontiers Biosci. 8, d264–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

