Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3
- PMID: 15741273
- PMCID: PMC554789
- DOI: 10.1073/pnas.0408376102
Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3
Abstract
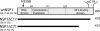
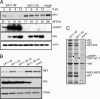
IFN regulatory factor 3 (IRF3), a constitutively expressed protein localizing largely to the cytoplasm, is a primary effector of the innate immune response. Infection can trigger the phosphorylation, dimerization, and nuclear translocation of IRF3, where the factor stimulates the expression and release of IFN. In this study, we determined that the rotavirus gene 5 product, nonstructural protein 1 (NSP1), interacts with IRF3 in the infected cell. To understand the importance of the interaction, we compared IRF3 activation by rotaviruses expressing wild-type and C-truncated forms of NSP1. The analysis showed that IRF3 underwent dimerization and nuclear translocation and stimulated IFN promoter activity in infected cells expressing truncated NSP1. In contrast, infected cells expressing wild-type NSP1 were characterized by the rapid degradation of IRF3 during the replication cycle, severe decreases in IRF3 dimerization and nuclear translocation, and lack of IFN promoter activity. The implication of these results, that wild-type NSP1 is an antagonist of the IFN-signaling pathway, was confirmed in transient expression assays, which showed that wild-type NSP1, but not the C-truncated protein, induced the degradation of IRF3 fusion proteins. Related experiments indicated that NSP1 mediates IRF3 degradation through a proteasome-dependent pathway. The critical role of NSP1 in promoting cell-to-cell spread of rotavirus was demonstrated by using gene 5-specific short interfering RNAs in plaque assays. Although several viruses have been described that subvert the innate immune response by preventing IRF3 activation, rotavirus is identified as one that accomplishes this task by inducing the degradation of IRF3.
Figures

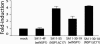


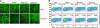

References
-
- Iwamura, T., Yoneyama, M., Yamaguchi, K., Suhara, W., Mori, W., Shiota, K., Okabe, Y., Namiki, H. & Fujita, T. (2001) Genes Cells 6, 375–388. - PubMed
-
- Hiscott, J., Pitha, P., Genin, P., Nguyen, H., Heylbroeck, C., Mamane, Y., Algarte, M. & Lin, R. (1999) J. Interf. Cytok. Res. 19, 1–13. - PubMed
-
- Sharma, S., tenOever, B. R., Grandvaux, N., Zhou, G. P., Lin, R. & Hiscott, J. (2003) Science 300, 1148–1151. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

