Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome
- PMID: 15741317
- PMCID: PMC551573
- DOI: 10.1101/gad.317405
Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome
Abstract
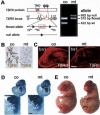
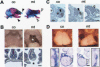
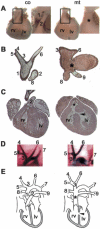
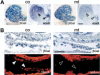
Specific inactivation of TGFbeta signaling in neural crest stem cells (NCSCs) results in cardiovascular defects and thymic, parathyroid, and craniofacial anomalies. All these malformations characterize DiGeorge syndrome, the most common microdeletion syndrome in humans. Consistent with a role of TGFbeta in promoting non-neural lineages in NCSCs, mutant neural crest cells migrate into the pharyngeal apparatus but are unable to acquire non-neural cell fates. Moreover, in neural crest cells, TGFbeta signaling is both sufficient and required for phosphorylation of CrkL, a signal adaptor protein implicated in the development of DiGeorge syndrome. Thus, TGFbeta signal modulation in neural crest differentiation might play a crucial role in the etiology of DiGeorge syndrome.
Figures
References
-
- Berti L., Mittler, G., Przemeck, G.K., Stelzer, G., Gunzler, B., Amati, F., Conti, E., Dallapiccola, B., Hrabe de Angelis, M., Novelli, G., et al. 2001. Isolation and characterization of a novel gene from the DiGeorge chromosomal region that encodes for a mediator subunit. Genomics 74: 320-332. - PubMed
-
- Brault V., Moore, R., Kutsch, S., Ishibashi, M., Rowitch, D.H., McMahon, A.P., Sommer, L., Boussida, O., and Kemler, R. 2001. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128: 1253-1264. - PubMed
-
- Chen S. and Lechleider, R.J. 2004. Transforming growth factor-β induced differentiation of smooth muscle from a neural crest stem cell line. Circ. Res. 94: 1195-1202. - PubMed
-
- Derynck R. and Zhang, Y.E. 2003. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577-584. - PubMed
-
- Feller S.M. 2001. Crk family adaptors—Signalling complex formation and biological roles. Oncogene 20: 6348-6371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
