L-Arginine increases the solubility of unfolded species of hen egg white lysozyme
- PMID: 15741330
- PMCID: PMC2253432
- DOI: 10.1110/ps.041085005
L-Arginine increases the solubility of unfolded species of hen egg white lysozyme
Abstract
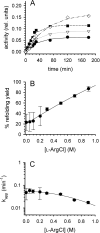
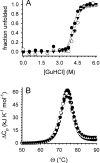
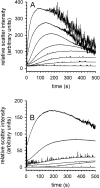
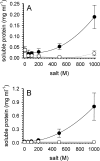
L-Arginine (L-Arg) has been widely used as an enhancer of protein renaturation. The mechanism behind its action is still not fully understood. Using hen egg white lysozyme as a model protein, we present data that clearly demonstrate the suppression of the aggregation of denatured protein by L-Arg. By chemical modification of free cysteines, a series of unfolded lysozyme species were obtained that served as models for unfolded and intermediate states during the process of oxidative refolding. An increased equilibrium solubility of unfolded species and intermediates in the presence of L-Arg seems to be its major mechanism of action.
Figures
Similar articles
-
L-argininamide improves the refolding more effectively than L-arginine.J Biotechnol. 2007 Jun 15;130(2):153-60. doi: 10.1016/j.jbiotec.2007.03.003. Epub 2007 Mar 12. J Biotechnol. 2007. PMID: 17434637
-
Equilibrium and kinetic folding of hen egg-white lysozyme under acidic conditions.Proteins. 2002 Dec 1;49(4):472-82. doi: 10.1002/prot.10215. Proteins. 2002. PMID: 12402357
-
Oxidative refolding of reduced, denatured lysozyme in AOT reverse micelles.J Colloid Interface Sci. 2008 Jun 1;322(1):95-103. doi: 10.1016/j.jcis.2008.02.057. Epub 2008 Mar 4. J Colloid Interface Sci. 2008. PMID: 18377920
-
Protein crystallization under high pressure.Biochim Biophys Acta. 2002 Mar 25;1595(1-2):345-56. doi: 10.1016/s0167-4838(01)00355-7. Biochim Biophys Acta. 2002. PMID: 11983407 Review.
-
Review: Why is arginine effective in suppressing aggregation?Protein Pept Lett. 2005 Oct;12(7):613-9. doi: 10.2174/0929866054696109. Protein Pept Lett. 2005. PMID: 16522173 Review.
Cited by
-
A simplified method for the efficient purification and refolding of recombinant human TRAIL.Biotechnol Prog. 2020 Sep;36(5):e3007. doi: 10.1002/btpr.3007. Epub 2020 May 11. Biotechnol Prog. 2020. PMID: 32329219 Free PMC article.
-
Studying the mechanism of phase separation in aqueous solutions of globular proteins via molecular dynamics computer simulations.Phys Chem Chem Phys. 2021 Jan 6;23(1):415-424. doi: 10.1039/d0cp05160h. Phys Chem Chem Phys. 2021. PMID: 33319872 Free PMC article.
-
Effects of solutes on solubilization and refolding of proteins from inclusion bodies with high hydrostatic pressure.Protein Sci. 2006 Feb;15(2):304-13. doi: 10.1110/ps.051813506. Epub 2005 Dec 29. Protein Sci. 2006. PMID: 16385003 Free PMC article.
-
Quantification of anti-aggregation activity of chaperones: a test-system based on dithiothreitol-induced aggregation of bovine serum albumin.PLoS One. 2013 Sep 10;8(9):e74367. doi: 10.1371/journal.pone.0074367. eCollection 2013. PLoS One. 2013. PMID: 24058554 Free PMC article.
-
A change in the aggregation pathway of bovine serum albumin in the presence of arginine and its derivatives.Sci Rep. 2017 Jun 21;7(1):3984. doi: 10.1038/s41598-017-04409-x. Sci Rep. 2017. PMID: 28638090 Free PMC article.
References
-
- Acharya, A.S. and Taniuchi, H. 1982. Implication of the structure and stability of disulfide intermediates of lysozyme on the mechanism of renaturation. Mol. Cell. Biochem. 44 129–148. - PubMed
-
- Arakawa, T. and Tsumoto, K. 2003. The effects of arginine on refolding of aggregated proteins: Not facilitate refolding, but suppress aggregation. Biochem. Biophys. Res. Commun. 304 148–152. - PubMed
-
- Asano, R., Kudo, T., Makabe, K., Tsumoto, K., and Kumagai, I. 2002. Antitumor activity of interleukin-21 prepared by novel refolding procedure from inclusion bodies expressed in Escherichia coli. FEBS Lett. 528 70–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

