The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL 12) is altered by pH, phosphate, sulfate, and heparin
- PMID: 15741341
- PMCID: PMC2253449
- DOI: 10.1110/ps.041219505
The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL 12) is altered by pH, phosphate, sulfate, and heparin
Abstract
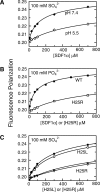
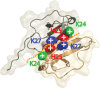
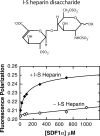
Chemokines, like stromal cell-derived factor-1 (SDF1/CXCL12), are small secreted proteins that signal cells to migrate. Because SDF1 and its receptor CXCR4 play important roles in embryonic development, cancer metastasis, and HIV/AIDS, this chemokine signaling system is the subject of intense study. However, it is not known whether the monomeric or dimeric structure of SDF1 is responsible for signaling in vivo. Previous structural studies portrayed the SDF1 structure as either strictly monomeric in solution or dimeric when crystallized. Here, we report two-dimensional NMR, pulsed-field gradient diffusion and fluorescence polarization measurements at various SDF1 concentrations, solution conditions, and pH. These results demonstrate that SDF1 can form a dimeric structure in solution, but only at nonacidic pH when stabilizing counterions are present. Thus, while the previous NMR structural studies were performed under acidic conditions that strongly promote the monomeric state, crystallographic studies used nonacidic buffer conditions that included divalent anions shown here to promote dimerization. This pH-sensitive aggregation behavior is explained by a dense cluster of positively charged residues at the SDF1 dimer interface that includes a histidine side chain at its center. A heparin disaccharide shifts the SDF1 monomer-dimer equilibrium in the same manner as other stabilizing anions, suggesting that glycosaminoglycan binding may be coupled to SDF1 dimerization in vivo.
Figures

Similar articles
-
Structural and functional basis of CXCL12 (stromal cell-derived factor-1 alpha) binding to heparin.J Biol Chem. 2007 Mar 30;282(13):10018-10027. doi: 10.1074/jbc.M608796200. Epub 2007 Jan 29. J Biol Chem. 2007. PMID: 17264079 Free PMC article.
-
Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12.Protein Sci. 2009 Jul;18(7):1359-69. doi: 10.1002/pro.167. Protein Sci. 2009. PMID: 19551879 Free PMC article.
-
Backbone dynamics of SDF-1alpha determined by NMR: interpretation in the presence of monomer-dimer equilibrium.Protein Sci. 2006 Nov;15(11):2568-78. doi: 10.1110/ps.062255806. Protein Sci. 2006. PMID: 17075134 Free PMC article.
-
Role of stromal cell-derived factor 1 (SDF1/CXCL12) in regulating anterior pituitary function.J Mol Endocrinol. 2007 Mar;38(3):383-9. doi: 10.1677/JME-06-0014. J Mol Endocrinol. 2007. PMID: 17339401 Review.
-
CXCL12-CXCR4/CXCR7 Axis in Colorectal Cancer: Therapeutic Target in Preclinical and Clinical Studies.Int J Mol Sci. 2021 Jul 9;22(14):7371. doi: 10.3390/ijms22147371. Int J Mol Sci. 2021. PMID: 34298991 Free PMC article. Review.
Cited by
-
The anti-cancer properties of heparin and its derivatives: a review and prospect.Cell Adh Migr. 2020 Dec;14(1):118-128. doi: 10.1080/19336918.2020.1767489. Cell Adh Migr. 2020. PMID: 32538273 Free PMC article. Review.
-
A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability.Mol Cancer Ther. 2012 Nov;11(11):2516-25. doi: 10.1158/1535-7163.MCT-12-0494. Epub 2012 Aug 6. Mol Cancer Ther. 2012. PMID: 22869557 Free PMC article.
-
Neutralizing endogenous chemokines with small molecules. Principles and potential therapeutic applications.Pharmacol Ther. 2010 Apr;126(1):39-55. doi: 10.1016/j.pharmthera.2009.12.003. Epub 2010 Feb 1. Pharmacol Ther. 2010. PMID: 20117133 Free PMC article. Review.
-
A role for the CXCR4-CXCL12 axis in the little skate, Leucoraja erinacea.Am J Physiol Regul Integr Comp Physiol. 2018 Aug 1;315(2):R218-R229. doi: 10.1152/ajpregu.00322.2017. Epub 2018 Apr 11. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29641231 Free PMC article.
-
The structural role of receptor tyrosine sulfation in chemokine recognition.Br J Pharmacol. 2014 Mar;171(5):1167-79. doi: 10.1111/bph.12455. Br J Pharmacol. 2014. PMID: 24116930 Free PMC article. Review.
References
-
- Altieri, A.S., Hinton, D.P., and Byrd, R.A. 1995. Association of biomolecular systems via pulsed field gradient NMR self-diffusion measurements. J. Am. Chem. Soc. 117 7566–7567.
-
- Amara, A., Lorthioir, O., Valenzuela, A., Magerus, A., Thelen, M., Montes, M., Virelizier, J.L., Delepierre, M., Baleux, F., Lortat-Jacob, H., et al. 1999. Stromal cell-derived factor-1α associates with heparan sulfates through the first β-strand of the chemokine. J. Biol. Chem. 274 23916–23925. - PubMed
-
- Bleul, C.C., Farzan, M., Choe, H., Parolin, C., Clark-Lewis, I., Sodroski, J., and Springer, T.A. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382 829–833. - PubMed
-
- Burrows, S.D., Doyle, M.L., Murphy, K.P., Franklin, S.G., White, J.R., Brooks, I., McNulty, D.E., Scott, M.O., Knutson, J.R., Porter, D., et al. 1994. Determination of the monomer–dimer equilibrium of interleukin-8 reveals it is a monomer at physiological concentrations. Biochemistry 33 12741–12745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

