Helix periodicity, topology, and dynamics of membrane-associated alpha-synuclein
- PMID: 15741347
- PMCID: PMC2253433
- DOI: 10.1110/ps.041255905
Helix periodicity, topology, and dynamics of membrane-associated alpha-synuclein
Abstract
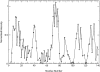
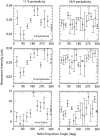
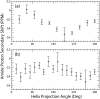
The protein alpha-Synuclein (aS) is a synaptic vesicle-associated regulator of synaptic strength and dopamine homeostasis with a pathological role in Parkinson's disease. The normal function of aS depends on a membrane-associated conformation that is adopted upon binding to negatively charged lipid surfaces. Previously we found that the membrane-binding domain of aS is helical and suggested that it may exhibit an unusual structural periodicity. Here we present a study of the periodicity, topology, and dynamics of detergent micelle-bound aS using paramagnetic spin labels embedded in the micelle or attached to the protein. We show that the helical region of aS completes three full turns every 11 residues, demonstrating the proposed 11/3 periodicity. We also find that the membrane-binding domain is partially buried in the micelle surface and bends toward the hydrophobic interior, but does not traverse the micelle. Deeper submersion of certain regions within the micelle, including the unique lysine-free sixth 11-residue repeat, is observed and may be functionally important. There are no long-range tertiary contacts within this domain, indicating a highly extended configuration. The backbone dynamics of the micelle-bound region are relatively uniform with a slight decrease in flexibility observed toward the C-terminal end. These results clarify the topological features of aS bound to membrane-mimicking detergent micelles, with implications for aS function and pathology.
Figures

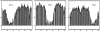

References
-
- Abeliovich, A., Schmitz, Y., Farinas, I., Choi-Lundberg, D., Ho, W.H., Castillo, P.E., Shinsky, N., Verdugo, J.M., Armanini, M., Ryan, A., et al. 2000. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25 239–252. - PubMed
-
- Ahn, B.H., Rhim, H., Kim, S.Y., Sung, Y.M., Lee, M.Y., Choi, J.Y., Wolozin, B., Chang, J.S., Lee, Y.H., Kwon, T.K., et al. 2002. α-Synuclein interacts with phospholipase D isozymes and inhibits pervanadate-induced phospholipase D activation in human embryonic kidney-293 cells. J. Biol. Chem. 277 12334–12342. - PubMed
-
- Bisaglia, M., Tessari, I., Pinato, L., Bellanda, M., Giraudo, S., Fasano, M., Bergantino, E., Bubacco, L., and Mammi, S. 2005. A topological model of the interaction between α-synuclein and sodium dodecyl sulfate micelles. Biochemistry 44 329–339. - PubMed
-
- Blundell, T., Barlow, D., Borkakoti, N., and Thornton, J. 1983. Solvent-induced distortions and the curvature of α-helices. Nature 306 281–283. - PubMed
-
- Bussell Jr., R. and Eliezer, D. 2001. Residual structure and dynamics in Parkinson’s disease-associated mutants of α-synuclein. J. Biol. Chem. 276 45996–46003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

