Interaction of the endothelial nitric oxide synthase with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport
- PMID: 15743275
- PMCID: PMC1134876
- DOI: 10.1042/BJ20041005
Interaction of the endothelial nitric oxide synthase with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport
Abstract
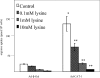
eNOS (endothelial nitric oxide synthase) catalyses the conversion of L-arginine into L-citrulline and NO. Evidence has been presented previously that eNOS is associated with the CAT (cationic amino acid transporter)-1 arginine transporter in endothelial caveolae, and it has been proposed that eNOS-CAT-1 association facilitates the delivery of extracellular L-arginine to eNOS. Definitive proof of a protein-protein interaction between eNOS and CAT-1 is lacking, however, and it is also unknown whether the two proteins interact directly or via an adaptor protein. In the present study, we raised a polyclonal antibody against CAT-1, and show using reciprocal co-immunoprecipitation protocols that eNOS and CAT-1 do indeed form a complex in BAECs (bovine aortic endothelial cells). In vitro binding assays with GST (glutathione S-transferase)-CAT-1 fusion proteins and eNOS show that the two proteins interact directly and that no single CAT-1 intracellular domain is sufficient to mediate the interaction. Overexpression of CAT-1 in BAECs by adenoviral-mediated gene transfer results in significant increases in both L-arginine uptake and NO production by the cells. However, whereas increased L-arginine transport is reversed completely by the CAT-1 inhibitor, L-lysine, increased NO release is unaltered, suggesting that NO production in this in vitro model is independent of CAT-1-mediated transport. Furthermore, eNOS enzymic activity is increased in lysates of CAT-1-overexpressing cells accompanied by increased phosphorylation of eNOS at Ser-1179 and Ser-635, and decreased association of eNOS with caveolin-1. Taken together, these data suggest that direct interaction of eNOS with CAT-1 enhances NO release by a mechanism not involving arginine transport.
Figures


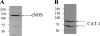


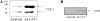

Similar articles
-
Reduced l-arginine transport and nitric oxide synthesis in human umbilical vein endothelial cells from intrauterine growth restriction pregnancies is not further altered by hypoxia.Placenta. 2009 Jul;30(7):625-33. doi: 10.1016/j.placenta.2009.04.010. Epub 2009 Jun 7. Placenta. 2009. PMID: 19501907
-
CAT-1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines.J Cell Biochem. 2011 Apr;112(4):1084-92. doi: 10.1002/jcb.23022. J Cell Biochem. 2011. PMID: 21308737
-
Cyclosporine attenuates arginine transport, in human endothelial cells, through modulation of cationic amino acid transporter-1.Am J Nephrol. 2013;37(6):613-9. doi: 10.1159/000350614. Epub 2013 Jun 13. Am J Nephrol. 2013. PMID: 23796541
-
Modulation of the L-arginine/nitric oxide signalling pathway in vascular endothelial cells.Biochem Soc Symp. 2004;(71):143-56. doi: 10.1042/bss0710143. Biochem Soc Symp. 2004. PMID: 15777019 Review.
-
A role for insulin on L-arginine transport in fetal endothelial dysfunction in hyperglycaemia.Curr Vasc Pharmacol. 2009 Oct;7(4):467-74. doi: 10.2174/157016109789043919. Curr Vasc Pharmacol. 2009. PMID: 19485892 Review.
Cited by
-
Extracellular l-arginine Enhances Relaxations Induced by Opening of Calcium-Activated SKCa Channels in Porcine Retinal Arteriole.Int J Mol Sci. 2019 Apr 25;20(8):2032. doi: 10.3390/ijms20082032. Int J Mol Sci. 2019. PMID: 31027156 Free PMC article.
-
Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1.Circ Res. 2008 Aug 15;103(4):360-8. doi: 10.1161/CIRCRESAHA.108.178897. Epub 2008 Jul 3. Circ Res. 2008. PMID: 18599867 Free PMC article.
-
Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism.Br J Clin Pharmacol. 2008 Jan;65(1):51-9. doi: 10.1111/j.1365-2125.2007.02990.x. Epub 2007 Jul 27. Br J Clin Pharmacol. 2008. PMID: 17662090 Free PMC article. Clinical Trial.
-
Nitric oxide: what's new to NO?Am J Physiol Cell Physiol. 2017 Mar 1;312(3):C254-C262. doi: 10.1152/ajpcell.00315.2016. Epub 2016 Dec 14. Am J Physiol Cell Physiol. 2017. PMID: 27974299 Free PMC article. Review.
-
Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery.Chem Rev. 2013 Jul 10;113(7):4633-79. doi: 10.1021/cr300163e. Epub 2013 Mar 20. Chem Rev. 2013. PMID: 23514336 Free PMC article. Review. No abstract available.
References
-
- Fleming I., Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1–R12. - PubMed
-
- Devés R., Boyd C. A. R. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol. Rev. 1998;78:487–545. - PubMed
-
- Zharikov S. I., Block E. R. Characterization of L-arginine uptake by plasma membrane vesicles isolated from cultured pulmonary artery endothelial cells. Biochim. Biophys. Acta. 1998;1369:173–183. - PubMed
-
- McDonald K. K., Zharikov S., Block E. R., Kilberg M. S. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J. Biol. Chem. 1997;272:31213–31216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

