Arthritis imaging using a near-infrared fluorescence folate-targeted probe
- PMID: 15743478
- PMCID: PMC1065321
- DOI: 10.1186/ar1483
Arthritis imaging using a near-infrared fluorescence folate-targeted probe
Abstract
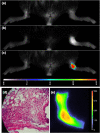
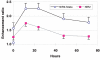
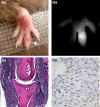
A recently developed near-infrared fluorescence-labeled folate probe (NIR2-folate) was tested for in vivo imaging of arthritis using a lipopolysaccharide intra-articular injection model and a KRN transgenic mice serum induction mouse model. In the lipopolysaccharide injection model, the fluorescence signal intensity of NIR2-folate (n = 12) and of free NIR2 (n = 5) was compared between lipopolysaccharide-treated and control joints. The fluorescence signal intensity of the NIR2-folate probe at the inflammatory joints was found to be significantly higher than the control normal joints (up to 2.3-fold, P < 0.001). The NIR2-free dye injection group showed a persistent lower enhancement ratio than the NIR2-folate probe injection group. Excessive folic acid was also given to demonstrate a competitive effect with the NIR2-folate. In the KRN serum transfer model (n = 4), NIR2-folate was applied at different time points after serum transfer, and the inflamed joints could be detected as early as 30 hours after arthritogenic antibody transfer (1.8-fold increase in signal intensity). Fluorescence microscopy, histology, and immunohistochemistry validated the optical imaging results. We conclude that in vivo arthritis detection was feasible using a folate-targeted near-infrared fluorescence probe. This receptor-targeted imaging method may facilitate improved arthritis diagnosis and early assessment of the disease progress by providing an in vivo characterization of active macrophage status in inflammatory joint diseases.
Figures

Similar articles
-
Enhanced tumor detection using a folate receptor-targeted near-infrared fluorochrome conjugate.Bioconjug Chem. 2003 May-Jun;14(3):539-45. doi: 10.1021/bc0340114. Bioconjug Chem. 2003. PMID: 12757377
-
In vivo imaging of experimental arthritis with near-infrared fluorescence.Arthritis Rheum. 2004 Mar;50(3):961-7. doi: 10.1002/art.20112. Arthritis Rheum. 2004. PMID: 15022340
-
A receptor-targeted near-infrared fluorescence probe for in vivo tumor imaging.Chembiochem. 2002 Aug 2;3(8):784-6. doi: 10.1002/1439-7633(20020802)3:8<784::AID-CBIC784>3.0.CO;2-X. Chembiochem. 2002. PMID: 12203978 No abstract available.
-
Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis.Adv Drug Deliv Rev. 2004 Apr 29;56(8):1205-17. doi: 10.1016/j.addr.2004.01.012. Adv Drug Deliv Rev. 2004. PMID: 15094216 Review.
-
Endothelial cells and macrophages, partners in atherosclerotic plaque progression.Arch Physiol Biochem. 2006 Oct-Dec;112(4-5):245-53. doi: 10.1080/13813450601094706. Arch Physiol Biochem. 2006. PMID: 17178598 Review.
Cited by
-
Synthesis and Preclinical Evaluation of Folate-NOTA-Al(18)F for PET Imaging of Folate-Receptor-Positive Tumors.Mol Pharm. 2016 May 2;13(5):1520-7. doi: 10.1021/acs.molpharmaceut.5b00989. Epub 2016 Apr 18. Mol Pharm. 2016. PMID: 27054811 Free PMC article.
-
Predicting Response to Therapy for Autoimmune and Inflammatory Diseases Using a Folate Receptor-Targeted Near-Infrared Fluorescent Imaging Agent.Mol Imaging Biol. 2016 Apr;18(2):201-8. doi: 10.1007/s11307-015-0876-y. Mol Imaging Biol. 2016. PMID: 26242480
-
Noninvasive multimodal fluorescence and magnetic resonance imaging of whole-organ intervertebral discs.Biomed Opt Express. 2021 May 7;12(6):3214-3227. doi: 10.1364/BOE.421205. eCollection 2021 Jun 1. Biomed Opt Express. 2021. PMID: 34221655 Free PMC article.
-
FlexPro MD, a Mixture of Krill Oil, Astaxanthin, and Hyaluronic Acid, Suppresses Lipopolysaccharide-Induced Inflammatory Cytokine Production Through Inhibition of NF-κB.J Med Food. 2016 Dec;19(12):1196-1203. doi: 10.1089/jmf.2016.3787. J Med Food. 2016. PMID: 27982753 Free PMC article.
-
Assessment of collagen-induced arthritis using cyanine 5.5 conjugated with hydrophobically modified glycol chitosan nanoparticles: correlation with 18F-fluorodeoxyglucose positron emission tomography data.Korean J Radiol. 2012 Jul-Aug;13(4):450-7. doi: 10.3348/kjr.2012.13.4.450. Epub 2012 Jun 18. Korean J Radiol. 2012. PMID: 22778567 Free PMC article.
References
-
- Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999;26:717–719. - PubMed
-
- Johnston RB., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988;318:747–752. - PubMed
-
- Kamen BA, Wang MT, Streckfuss AJ, Peryea X, Anderson RG. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem. 1988;263:13602–13609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

