Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function
- PMID: 15743486
- PMCID: PMC1065334
- DOI: 10.1186/ar1499
Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function
Abstract
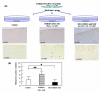
Oxidative stress leads to increased risk for osteoarthritis (OA) but the precise mechanism remains unclear. We undertook this study to clarify the impact of oxidative stress on the progression of OA from the viewpoint of oxygen free radical induced genomic instability, including telomere instability and resulting replicative senescence and dysfunction in human chondrocytes. Human chondrocytes and articular cartilage explants were isolated from knee joints of patients undergoing arthroplastic knee surgery for OA. Oxidative damage and antioxidative capacity in OA cartilage were investigated in donor-matched pairs of intact and degenerated regions of tissue isolated from the same cartilage explants. The results were histologically confirmed by immunohistochemistry for nitrotyrosine, which is considered to be a maker of oxidative damage. Under treatment with reactive oxygen species (ROS; 0.1 micromol/l H2O2) or an antioxidative agent (ascorbic acid: 100.0 micromol/l), cellular replicative potential, telomere instability and production of glycosaminoglycan (GAG) were assessed in cultured chondrocytes. In tissue cultures of articular cartilage explants, the presence of oxidative damage, chondrocyte telomere length and loss of GAG to the medium were analyzed in the presence or absence of ROS or ascorbic acid. Lower antioxidative capacity and stronger staining of nitrotyrosine were observed in the degenerating regions of OA cartilages as compared with the intact regions from same explants. Immunostaining for nitrotyrosine correlated with the severity of histological changes to OA cartilage, suggesting a correlation between oxidative damage and articular cartilage degeneration. During continuous culture of chondrocytes, telomere length, replicative capacity and GAG production were decreased by treatment with ROS. In contrast, treatment with an antioxidative agent resulted in a tendency to elongate telomere length and replicative lifespan in cultured chondrocytes. In tissue cultures of cartilage explants, nitrotyrosine staining, chondrocyte telomere length and GAG remaining in the cartilage tissue were lower in ROS-treated cartilages than in control groups, whereas the antioxidative agent treated group exhibited a tendency to maintain the chondrocyte telomere length and proteoglycan remaining in the cartilage explants, suggesting that oxidative stress induces chondrocyte telomere instability and catabolic changes in cartilage matrix structure and composition. Our findings clearly show that the presence of oxidative stress induces telomere genomic instability, replicative senescence and dysfunction of chondrocytes in OA cartilage, suggesting that oxidative stress, leading to chondrocyte senescence and cartilage ageing, might be responsible for the development of OA. New efforts to prevent the development and progression of OA may include strategies and interventions aimed at reducing oxidative damage in articular cartilage.
Figures





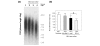
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

