Knockdown of c-Myc expression by RNAi inhibits MCF-7 breast tumor cells growth in vitro and in vivo
- PMID: 15743499
- PMCID: PMC1064129
- DOI: 10.1186/bcr975
Knockdown of c-Myc expression by RNAi inhibits MCF-7 breast tumor cells growth in vitro and in vivo
Abstract
Introduction: Breast cancer is the leading cause of cancer death in women worldwide. Elevated expression of c-Myc is a frequent genetic abnormality seen in this malignancy. For a better understanding of its role in maintaining the malignant phenotype, we used RNA interference (RNAi) directed against c-Myc in our study. RNAi provides a new, reliable method to investigate gene function and has the potential for gene therapy. The aim of the study was to examine the anti-tumor effects elicited by a decrease in the protein level of c-Myc by RNAi and its possible mechanism of effects in MCF-7 cells.
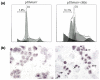
Method: A plasmid-based polymerase III promoter system was used to deliver and express short interfering RNA (siRNA) targeting c-myc to reduce its expression in MCF-7 cells. Western blot analysis was used to measure the protein level of c-Myc. We assessed the effects of c-Myc silencing on tumor growth by a growth curve, by soft agar assay and by nude mice experiments in vivo. Standard fluorescence-activated cell sorter analysis and TdT-mediated dUTP nick end labelling assay were used to determine apoptosis of the cells.
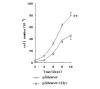
Results: Our data showed that plasmids expressing siRNA against c-myc markedly and durably reduced its expression in MCF-7 cells by up to 80%, decreased the growth rate of MCF-7 cells, inhibited colony formation in soft agar and significantly reduced tumor growth in nude mice. We also found that depletion of c-Myc in this manner promoted apoptosis of MCF-7 cells upon serum withdrawal.
Conclusion: c-Myc has a pivotal function in the development of breast cancer. Our data show that decreasing the c-Myc protein level in MCF-7 cells by RNAi could significantly inhibit tumor growth both in vitro and in vivo, and imply the therapeutic potential of RNAi on the treatment of breast cancer by targeting overexpression oncogenes such as c-myc, and c-myc might be a potential therapeutic target for human breast cancer.
Figures



Similar articles
-
Knockdown of the c-Jun-N-terminal kinase expression by siRNA inhibits MCF-7 breast carcinoma cell line growth.Oncol Rep. 2010 Nov;24(5):1339-45. doi: 10.3892/or_00000991. Oncol Rep. 2010. PMID: 20878129
-
Tumor-specific RNAi targeting eIF4E suppresses tumor growth, induces apoptosis and enhances cisplatin cytotoxicity in human breast carcinoma cells.Breast Cancer Res Treat. 2009 Feb;113(3):443-56. doi: 10.1007/s10549-008-9956-x. Epub 2008 Mar 10. Breast Cancer Res Treat. 2009. PMID: 18327707
-
[Inhibition of proliferation in MCF-7 breast cancer cells by plasmid-based siRNA targeting to oncogene c-myc].Sichuan Da Xue Xue Bao Yi Xue Ban. 2008 May;39(3):373-7. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008. PMID: 18575318 Chinese.
-
[Small interference RNAs directed against KDR gene inhibit the proliferation of breast cancer cells in vitro and in vivo].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008 Jan;24(1):58-61. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008. PMID: 18177622 Review. Chinese.
-
[In vivo siRNA delivery to tumor cells and its application to cancer gene therapy].Yakugaku Zasshi. 2007 Oct;127(10):1525-31. doi: 10.1248/yakushi.127.1525. Yakugaku Zasshi. 2007. PMID: 17917414 Review. Japanese.
Cited by
-
The adenoviral E1A N-terminal domain represses MYC transcription in human cancer cells by targeting both p300 and TRRAP and inhibiting MYC promoter acetylation of H3K18 and H4K16.Genes Cancer. 2016 Mar;7(3-4):98-109. doi: 10.18632/genesandcancer.99. Genes Cancer. 2016. PMID: 27382434 Free PMC article.
-
MYC regulates a pan-cancer network of co-expressed oncogenic splicing factors.Cell Rep. 2022 Nov 22;41(8):111704. doi: 10.1016/j.celrep.2022.111704. Cell Rep. 2022. PMID: 36417849 Free PMC article.
-
CRISPR/Cas9 screen for genome-wide interrogation of essential MYC-bound E-boxes in cancer cells.Mol Oncol. 2023 Nov;17(11):2295-2313. doi: 10.1002/1878-0261.13493. Epub 2023 Aug 7. Mol Oncol. 2023. PMID: 37519063 Free PMC article.
-
Multiscale Simulation as a Framework for the Enhanced Design of Nanodiamond-Polyethylenimine-based Gene Delivery.J Phys Chem Lett. 2012 Dec 4;3(24):3791-3797. doi: 10.1021/jz301756e. J Phys Chem Lett. 2012. PMID: 23304428 Free PMC article.
-
Nuclear reprogramming of luminal-like breast cancer cells generates Sox2-overexpressing cancer stem-like cellular states harboring transcriptional activation of the mTOR pathway.Cell Cycle. 2013 Sep 15;12(18):3109-24. doi: 10.4161/cc.26173. Epub 2013 Aug 21. Cell Cycle. 2013. PMID: 23974095 Free PMC article.
References
-
- WHO . World Health Report 2003. Geneva: World Health Organization; 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

