Methodological bias in cluster randomised trials
- PMID: 15743523
- PMCID: PMC554774
- DOI: 10.1186/1471-2288-5-10
Methodological bias in cluster randomised trials
Abstract
Background: Cluster randomised trials can be susceptible to a range of methodological problems. These problems are not commonly recognised by many researchers. In this paper we discuss the issues that can lead to bias in cluster trials.
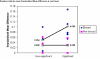
Methods: We used a sample of cluster randomised trials from a recent review and from a systematic review of hip protectors. We compared the mean age of participants between intervention groups in a sample of 'good' cluster trials with a sample of potentially biased trials. We also compared the effect sizes, in a funnel plot, between hip protector trials that used individual randomisation compared with those that used cluster randomisation.
Results: There is a tendency for cluster trials, with evidence methodological biases, to also show an age imbalance between treatment groups. In a funnel plot we show that all cluster trials show a large positive effect of hip protectors whilst individually randomised trials show a range of positive and negative effects, suggesting that cluster trials may be producing a biased estimate of effect.
Conclusion: Methodological biases in the design and execution of cluster randomised trials is frequent. Some of these biases associated with the use of cluster designs can be avoided through careful attention to the design of cluster trials. Firstly, if possible, individual allocation should be used. Secondly, if cluster allocation is required, then ideally participants should be identified before random allocation of the clusters. Third, if prior identification is not possible, then an independent recruiter should be used to recruit participants.
Figures
References
-
- Lindqust EF. Statistical Analysis in Educational Research. Houghton Mifflin Co, New York; 1940.
-
- Donner A, Klar NS. Design and Analysis of Cluster Randomisation Trials in Health Research. Hodder Arnold London; 2000.
-
- Schulz KF. Subverting randomisation in controlled trials. JAMA. 1995. pp. 456–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical